Manufacturing and Analytical Characterization - Biomolecular
(M1330-02-10) Direct and Automated Multi-Attribute Testing of Biologic Formulation with Benchtop NMR

Valentin Poirier, PhD
Solution Product Manager
Bruker BioSpin
Wissembourg Cedex, Alsace, France
Valentin Poirier, PhD
Solution Product Manager
Bruker BioSpin
Wissembourg Cedex, Alsace, France
Presenting Author(s)
Main Author(s)
Methods: Benchtop proton NMR data were acquired using a Fourier 80 spectrometer operating at 80 MHz. An optimized pulse sequence (noesycpdcgpps1d) integrating NOESY-based water presaturation and CPMG T2 filtering blocks was used to effectively suppress water and protein signals. An automated script dynamically optimized the water suppression frequency to ensure consistent spectral quality without manual intervention during acquisition. Data processing and analysis were fully automated, employing an external reference calibration workflow based on the PULCON methodology. Pure water served as the external reference for single-point calibration in each analytical run. NMR data were processed using a standardized script optimized for this application, ensuring consistent phase and baseline correction. Quantitative analyses were performed under full automation using the Advanced Chemical Profiling software, with excipient concentrations calculated directly from the single-point calibration. Correction factors, established during method development, were directly applied to adjust for the specific pulse sequence and short recycle delay. Samples consisted of model formulations containing excipients commonly used in biologics: organic buffers (histidine), cryoprotectants (sucrose, trehalose, mannitol), antioxidants (methionine), surfactants (polysorbate 20, polysorbate 80, poloxamer 188), and, optionally, viscosity reducers (arginine). All analyses were performed in at least triplicate, with performances assessed by recovery percentages and relative standard deviations
Results: We present selected results demonstrating the general performance of the platform for multi-attribute testing under full automation. In a first set of examples, we highlight the applicability of the method to the three main surfactants currently used in the market (polysorbate 20, polysorbate 80, poloxamer 188) within the typical 0.2–0.8 mg/mL range, in model formulations also containing histidine buffer (15–18 mM), methionine (10–12 mM), and sucrose (220–260 mM). The procedure yielded linear, accurate, and precise results across the studied ranges, with unit slopes, recoveries consistently between 90–110%, and relative standard deviations below 5% for all three surfactants. A second set of examples showcases the multi-attribute capabilities and robustness of the method, using a model mixture containing P188 (0.3–0.6 mg/mL), methionine (8–12 mM), histidine buffer (14–22 mM), and trehalose (0–320 mM). Two independent series of six concentration levels in trehalose were analyzed across three instruments. Using average recovery as the metric for each target, the method demonstrated consistent accuracy, with average recoveries in the 93–104% range. For trehalose, we further show excellent repeatability and intermediate precision across levels and instruments, with standard deviations below 1% and 3%, respectively.
Conclusion:
This study successfully demonstrates the capabilities of benchtop NMR-based analytical procedures for automated, accurate, and precise multi-attribute testing of biological formulations. The method effectively quantifies the panel of organic excipients typically present in such formulations, including critical and challenging surfactants. It achieves performance in terms of accuracy and precision that compares favorably with conventional chromatographic methods, while replacing multiple independent assays with a single, automated procedure. Moreover, it eliminates the need for sample preparation. Overall, this approach provides a simpler and faster methodology, enabling development and quality control laboratories to reduce turnaround times and minimize the number of analytical procedures required for biologic formulation testing.
References: R.G. Strickley, W.J. Lambert, Journal of Pharmaceutical Sciences 110 (2021) 2590−2608
C.A. Paschen, D. Klemm, T. Graf et al., Journal of Pharmaceutical and Biomedical Analysis 192 (2021) 113640
A. Martos et al., Journal of Pharmaceutical Sciences 109 (2020) 646-655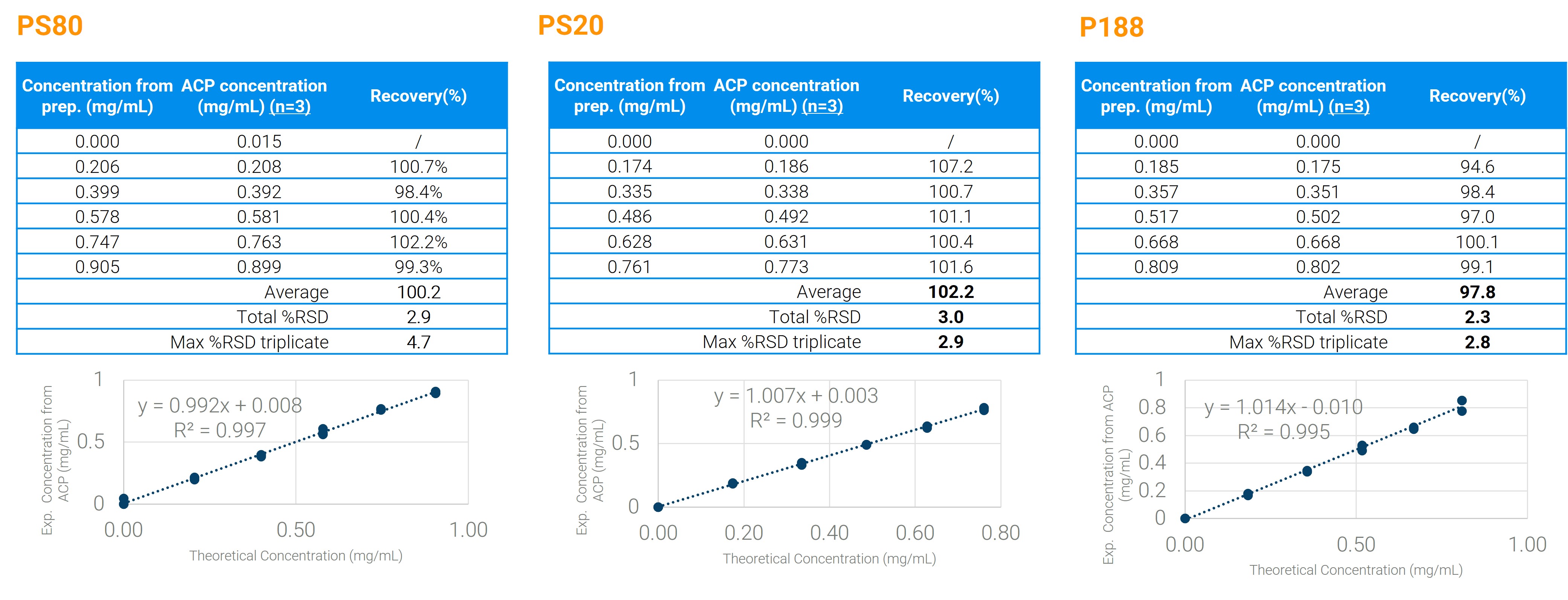
Figure 1: Example results obtained for the three main surfactants currently used in the market, using 6 levels of surfactant content in model formulations. Each formulation also contained sucrose, histidine, and methionine as additional excipients. For each concentration level, the average measured concentration and corresponding recovery from triplicate measurements are reported. Total %RSD represents the overall relative standard deviation across the entire dataset for the series. Max %RSD triplicate refers to the highest relative standard deviation observed among the individual triplicate measurements.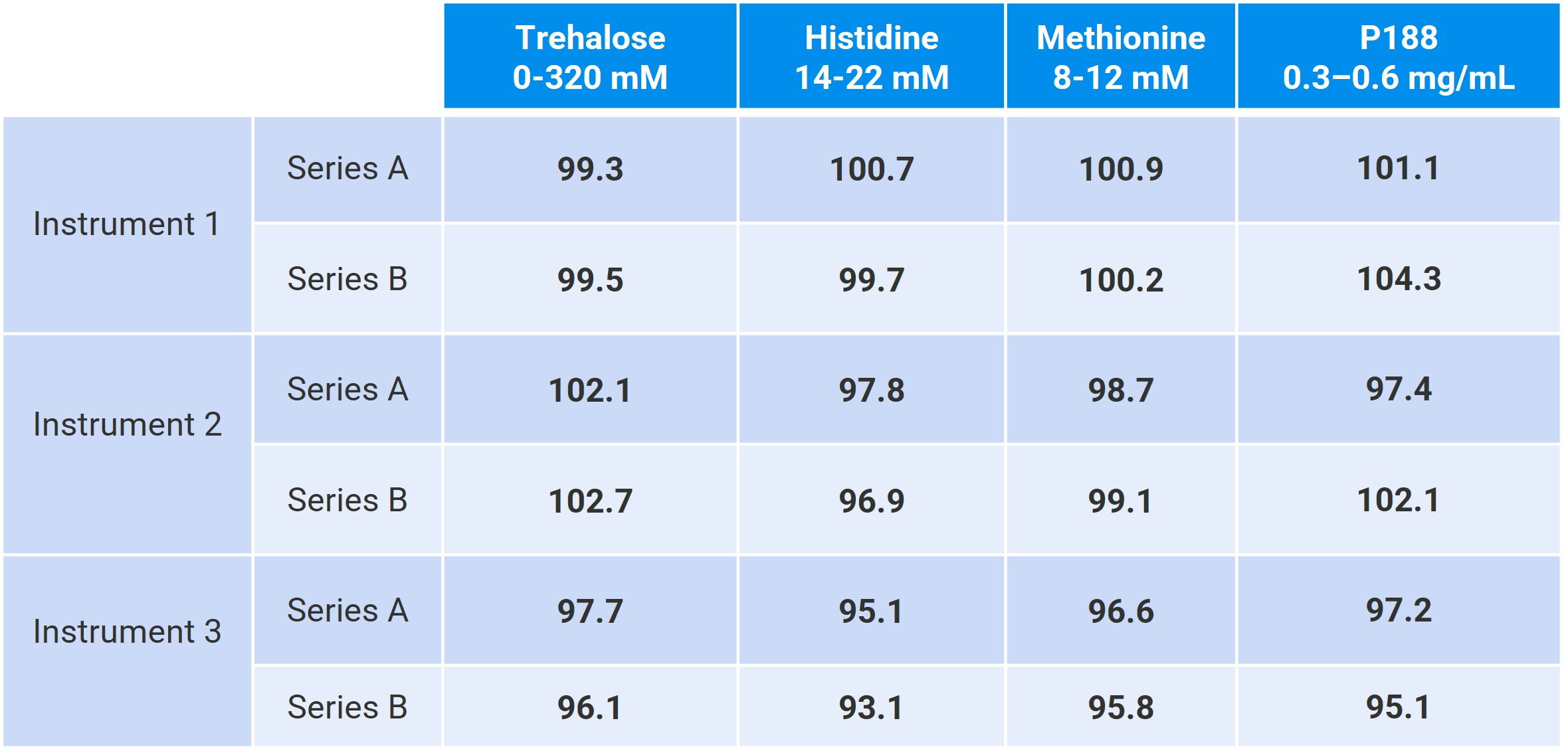
Table 1: Average recoveries obtained using two independent six-level series of model formulations analyzed across three instruments. Each preparation was measured four times, resulting in 24 data points per series and per instrument. All results were obtained under full automation using a single-point external calibration.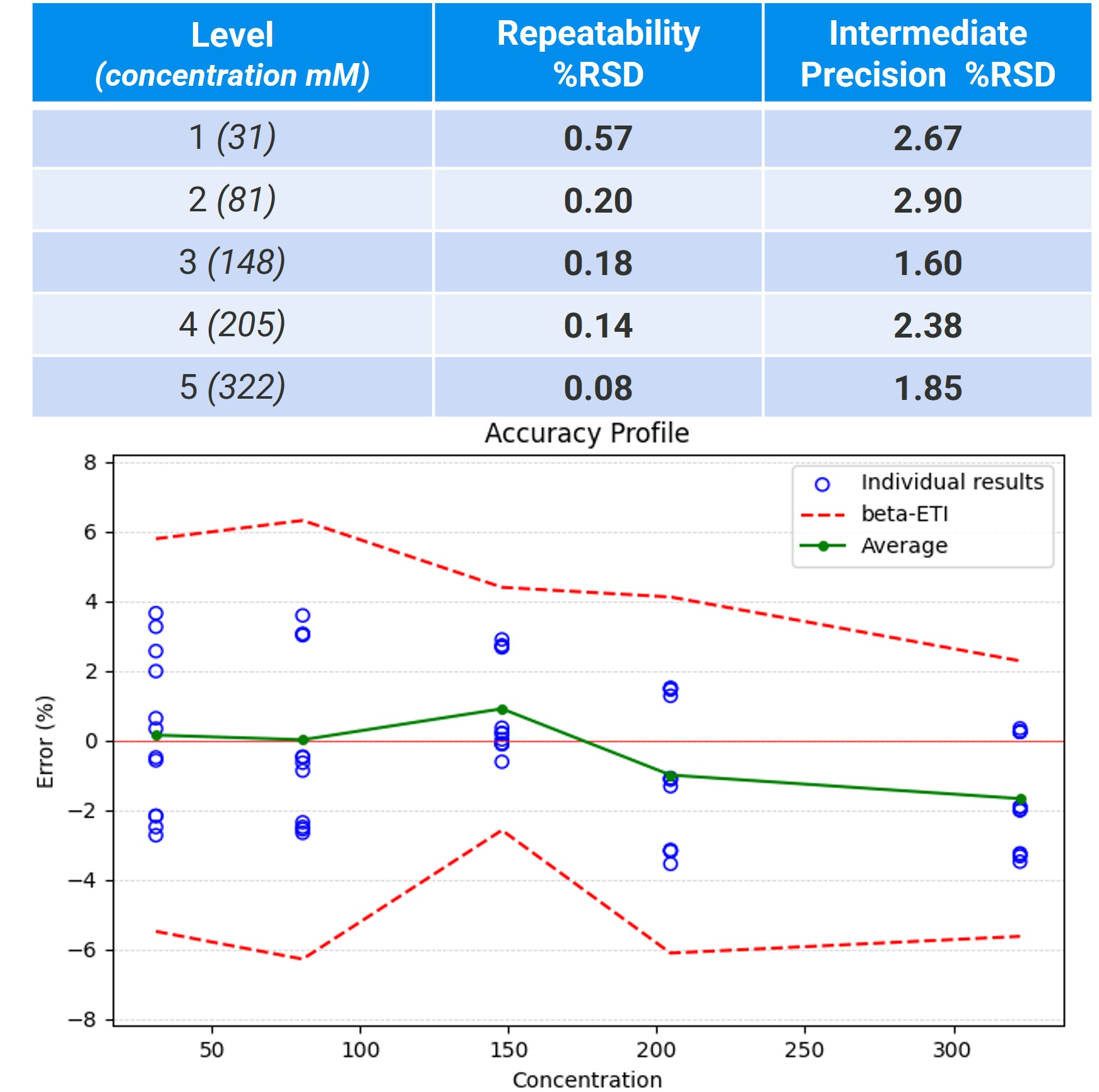
Figure 2: Detailed statistical analysis of trehalose in Series A (as presented in Table 1), showing for each concentration level the repeatability and intermediate precision expressed as relative standard deviations. The corresponding accuracy profile is reported as percentage error, using a β-expectation tolerance interval (beta-ETI) of 80%.
