Formulation and Delivery - Chemical
(T0930-07-44) In Vitro Deposition Studies of Microparticulate Nasal Powders
Tuesday, November 11, 2025
9:30 AM - 10:30 AM CT

Georgeta Caraua, MS (she/her/hers)
PhD Student
Universita Di Parma
Parma, Lombardia, Italy
Paolo Colombo, Ph.D.
Prof. Emeritus
University of Parma
Parma, Emilia-Romagna, Italy
Presenting Author(s)
Main Author(s)
Purpose: Loco-regional nasal delivery represents a targeted approach for administering different drugs, such as antivirals or anti-inflammatory, to the upper respiratory tract [1]. Nasal powder delivery usually is performed by insufflation with active dry powder devices. Recently, nasal powder delivery by inspiration has elicited particular attention because of the possibility to involve a larger surface deposition of the aerosol particles on the upper airways epithelial. Several factors, related to powder formulation and devices, influence the delivery of nasal aerosols produced [2]. In vitro aerosol particle deposition in upper airways can be assets using nasal cast models and/or Alberta Idealized Nasal Inlet connected to Next Generation Impactor (NGI). The intent is to establish the aerodynamic quality of the nasal powder [3]. The aim of this study was to evaluate the deposition behavior of a microparticulate nasal powders by means of TurbospinⓇ dry powder inhaler, activated by a sharp nasal breath intake (sniff). The inspiration performance was compared with the active nasal powder device, MiatⓇ, applying two different airflow rates to quantify the site deposition distribution with the two nasal devices.
Methods: AINI model was coupled with Next Generation Impactor (Figure 1A) with both devices at two air flow rates: 15 l/min and 34 l/min. Devices were placed into the AINI nostril with an accurately defined insertion depth of 15 mm and a delivery angle (horizontal plane) of 45°. The aerosolization test was performed on two pierced hard capsules loaded with 25 mg of powder. The test was performed in triplicate. In vitro deposition of the powder formulation was also performed in a realistic nasal cast (Inserm 1100, CEPR, Univ. of Tours, France) (Figure 1B) using with both devices at the two air flow rates mentioned above. The device set up applied was the same used for the AINI deposition study. The different nasal regions of interest were rinsed with appropriate volumes of water and the samples were quantified spectrophotometrically at 360 nm using a calibration curve ranging from 1 to 60 µg/mL.
Results: The nasal deposition studies performed with AINI model by means of TurbospinⓇ and MiatⓇ devices show different distribution of the powder in nasal passage sections: around 20 % and 40 %, respectively, in the nostrils area; around 20% and 10 %, respectively, in the nasopharynx area. Both devices showed similar deposition behavior in the turbinate’s (around 40%) and olfactory region (around 5 %). By performing the deposition studies with the nasal cast, the two devices performed a similar distribution of the powder in the nostril’s areas (around 50 %), turbinate’s area (around 40%) and olfactory region (around 5%). On the contrary, higher amount of powder deposited in the nasopharynx and trachea regions was observed for TurbospinⓇ DPI (around 5% and 10%, respectively) compared to Miat device ( < 1%). For both nasal models (AINI and Nasal Cast), no considerable differences were observed changing the air flow rates.
Conclusion: The deposition studies reveal the suitability of TurbospinⓇ device for a breath intake nasal dry powder inhaler at both basal (15 L/min) and sharp (34 L/min) air flow inspiration conditions. Indeed, with TurbospinⓇ it was obtained a distribution of the powder not only on the upper airways, but also on lower airways, covering all the potential virus infection sites. However, differences in deposition on nostril, nasopharynx, trachea and lungs region were observed comparing the two nasal models, probably related to the adhesion of the powder to the cavity (different nasal models’ materials) and/or to the different surface area of the models i.e. the idealized model with a single nostril and the full nasal geometry model.
References: [ ] Tiozzo Fasiolo L, Manniello MD, Tratta E, et al. Opportunity and challenges of nasal powders: Drug formulation and delivery. Eur J Pharm Sci. 2018;113:2-17. doi:10.1016/j.ejps.2017.09.027
[2] Le Guellec S, Ehrmann S, Vecellio L. In vitro - in vivo correlation of intranasal drug deposition. Adv Drug Deliv Rev. 2021;170:340-352. doi:10.1016/j.addr.2020.09.002
[3] Williams, Gerallt, and Julie D. Suman. 2022. "In Vitro Anatomical Models for Nasal Drug Delivery" Pharmaceutics 14, no. 7: 1353. doi.org/10.3390/pharmaceutics14071353
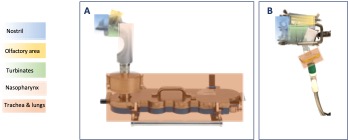
A) Experimental set-up of AINI model and nasal regions used for quantification. B) Experimental set-up of the nasal cast and nasal regions used for quantification.
Methods: AINI model was coupled with Next Generation Impactor (Figure 1A) with both devices at two air flow rates: 15 l/min and 34 l/min. Devices were placed into the AINI nostril with an accurately defined insertion depth of 15 mm and a delivery angle (horizontal plane) of 45°. The aerosolization test was performed on two pierced hard capsules loaded with 25 mg of powder. The test was performed in triplicate. In vitro deposition of the powder formulation was also performed in a realistic nasal cast (Inserm 1100, CEPR, Univ. of Tours, France) (Figure 1B) using with both devices at the two air flow rates mentioned above. The device set up applied was the same used for the AINI deposition study. The different nasal regions of interest were rinsed with appropriate volumes of water and the samples were quantified spectrophotometrically at 360 nm using a calibration curve ranging from 1 to 60 µg/mL.
Results: The nasal deposition studies performed with AINI model by means of TurbospinⓇ and MiatⓇ devices show different distribution of the powder in nasal passage sections: around 20 % and 40 %, respectively, in the nostrils area; around 20% and 10 %, respectively, in the nasopharynx area. Both devices showed similar deposition behavior in the turbinate’s (around 40%) and olfactory region (around 5 %). By performing the deposition studies with the nasal cast, the two devices performed a similar distribution of the powder in the nostril’s areas (around 50 %), turbinate’s area (around 40%) and olfactory region (around 5%). On the contrary, higher amount of powder deposited in the nasopharynx and trachea regions was observed for TurbospinⓇ DPI (around 5% and 10%, respectively) compared to Miat device ( < 1%). For both nasal models (AINI and Nasal Cast), no considerable differences were observed changing the air flow rates.
Conclusion: The deposition studies reveal the suitability of TurbospinⓇ device for a breath intake nasal dry powder inhaler at both basal (15 L/min) and sharp (34 L/min) air flow inspiration conditions. Indeed, with TurbospinⓇ it was obtained a distribution of the powder not only on the upper airways, but also on lower airways, covering all the potential virus infection sites. However, differences in deposition on nostril, nasopharynx, trachea and lungs region were observed comparing the two nasal models, probably related to the adhesion of the powder to the cavity (different nasal models’ materials) and/or to the different surface area of the models i.e. the idealized model with a single nostril and the full nasal geometry model.
References: [ ] Tiozzo Fasiolo L, Manniello MD, Tratta E, et al. Opportunity and challenges of nasal powders: Drug formulation and delivery. Eur J Pharm Sci. 2018;113:2-17. doi:10.1016/j.ejps.2017.09.027
[2] Le Guellec S, Ehrmann S, Vecellio L. In vitro - in vivo correlation of intranasal drug deposition. Adv Drug Deliv Rev. 2021;170:340-352. doi:10.1016/j.addr.2020.09.002
[3] Williams, Gerallt, and Julie D. Suman. 2022. "In Vitro Anatomical Models for Nasal Drug Delivery" Pharmaceutics 14, no. 7: 1353. doi.org/10.3390/pharmaceutics14071353

A) Experimental set-up of AINI model and nasal regions used for quantification. B) Experimental set-up of the nasal cast and nasal regions used for quantification.
