Formulation and Delivery - Chemical
(T1530-07-42) Leveraging Plant-Derived Extracellular Vesicles for Advanced Therapeutic Applications
Tuesday, November 11, 2025
3:30 PM - 4:30 PM CT
- HJ
Hillary Jean-pierre, BS
Graduate Assistant
University of Florida
Gainesville, Florida, United States - HJ
Hillary Jean-pierre, BS
Graduate Assistant
University of Florida
Gainesville, Florida, United States
Presenting Author(s)
Main Author(s)
Purpose: Extracellular vesicles(EVs) have gained attention in the field of Drug Delivery for their promising therapeutic potential due to their low immunogenicity, biological compatibility, and targeted delivery. In parallel, plant-derived extracellular vesicles (PDEVs) have recently gained attention for treating various gastrointestinal diseases and enhancing human well-being. The potential of PDEVs and characteristic properties remains underexplored, due to ineffective purification methods and limited research into outcomes of non-gastrointestinal illnesses. To address the lack of PDEV isolation standardization, we introduce an optimal isolation protocol for PDEVs. To demonstrate comparative drug delivery and therapeutic potential, we explored the cargo loading abilities as well as cellular uptake with mammalian cells using PDEVs, which lays the foundation for developing a novel and promising PDEV therapy.
Methods: To assess the optimization of PDEV isolation, we developed a robust methodology for isolating extracellular vesicles from Citrus Sinensis, Citrullus lanatus, Brassica oleracea, Brassica oleracea, and Capsicum annuum via ultracentrifugation, subsequently characterizing them through transmission electron microscopy (TEM) and nanoparticle tracking analysis (NTA). To demonstrate cellular uptake of PDEVs in mammalian cells, Citrus Sinensis-derived Extracellular vesicles (CSEVs) were labeled with BioTracker 555 Orange Dye and incubated with Medical Research Council cell strain 5 (MRC-5). cells at various time points (2.5 hours, 4.5 hours, and 6.5 hours), followed by fixation and imaging using light microscopy. To demonstrate comparative drug delivery and therapeutic potential, we explored the cargo-loading abilities of Citrullus lanatusas via a microfluidic device as well as cellular uptake with mammalian cells using PDEVs.
Results: A robust methodology for isolating various plant-derived extracellular vesicles (PDEVs) via ultracentrifugation was developed and subsequently characterized through transmission electron microscopy (90-170 nm) and nanoparticle tracking analysis (1.0 x 10^9 - 1.0 x 10^11 particles/mL). Notable cellular uptake of CSEVs by MRC-5 cells across all time points, with an initial uptake percentage of 44.37% at 2.5 hrs, peaking at 50.33% by 4.5 hrs, and declining to 25.93% at 6.5 hrs. This study showcases the rapid uptake of CSEVs by MRC-5 cells within 2.5 hours, reaching optimal uptake at 4.5 hours.
Conclusion: These results underscore the potential of PDEVs as a viable drug delivery system for diverse diseases. As an alternative to mammalian EVs, PDEVs have the advantages of easy and inexpensive access, the possibility of large-scale production, and the elimination of culture systems. Future investigations should delve into comparing the drug-loading capabilities of PDEVs with both standalone and mammalian-derived EVs, shedding light on their comparative efficacy and therapeutic potential.
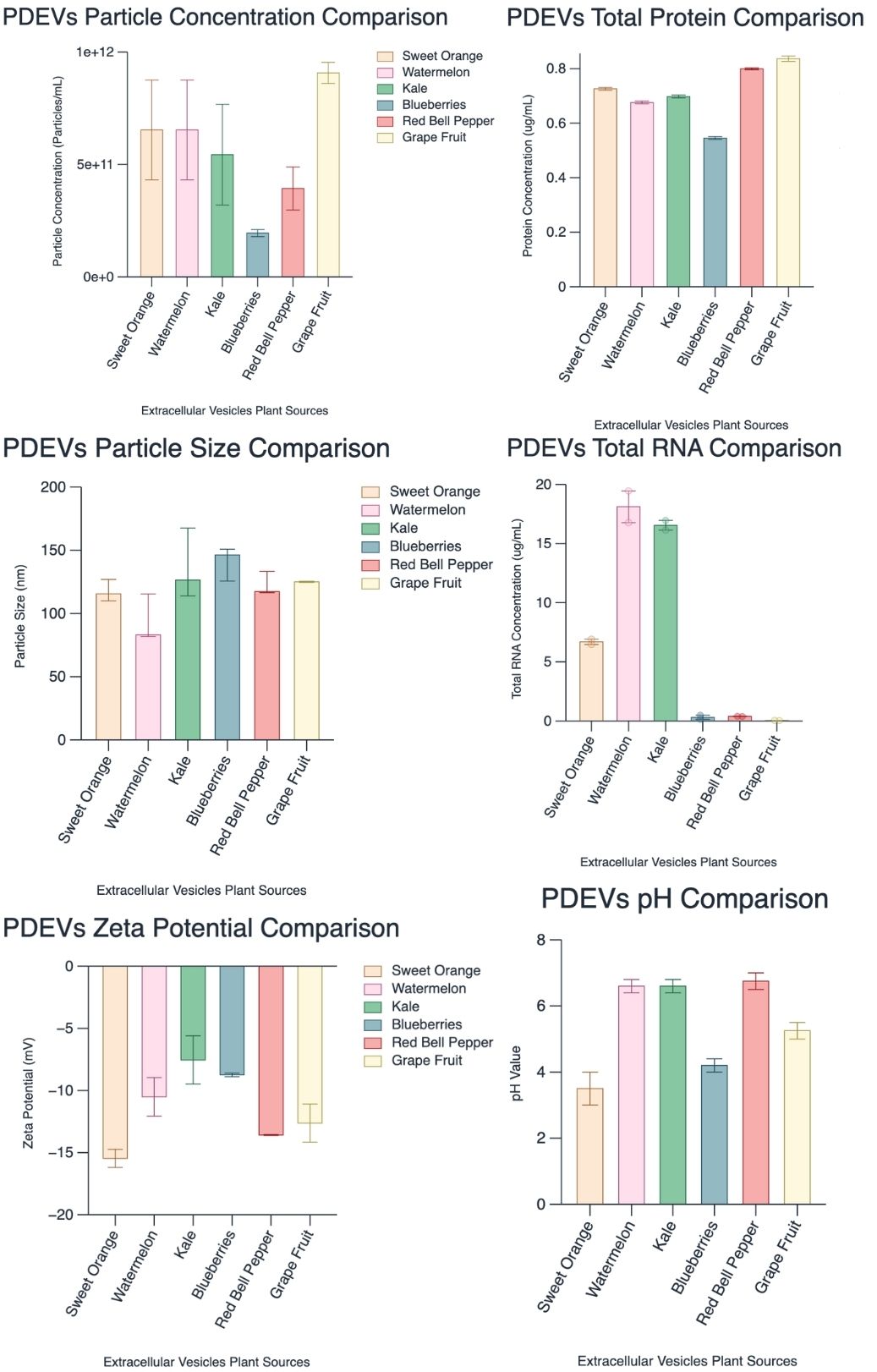
This Figure compares standard characterization methods for various plant-derived extracellular vesicles.
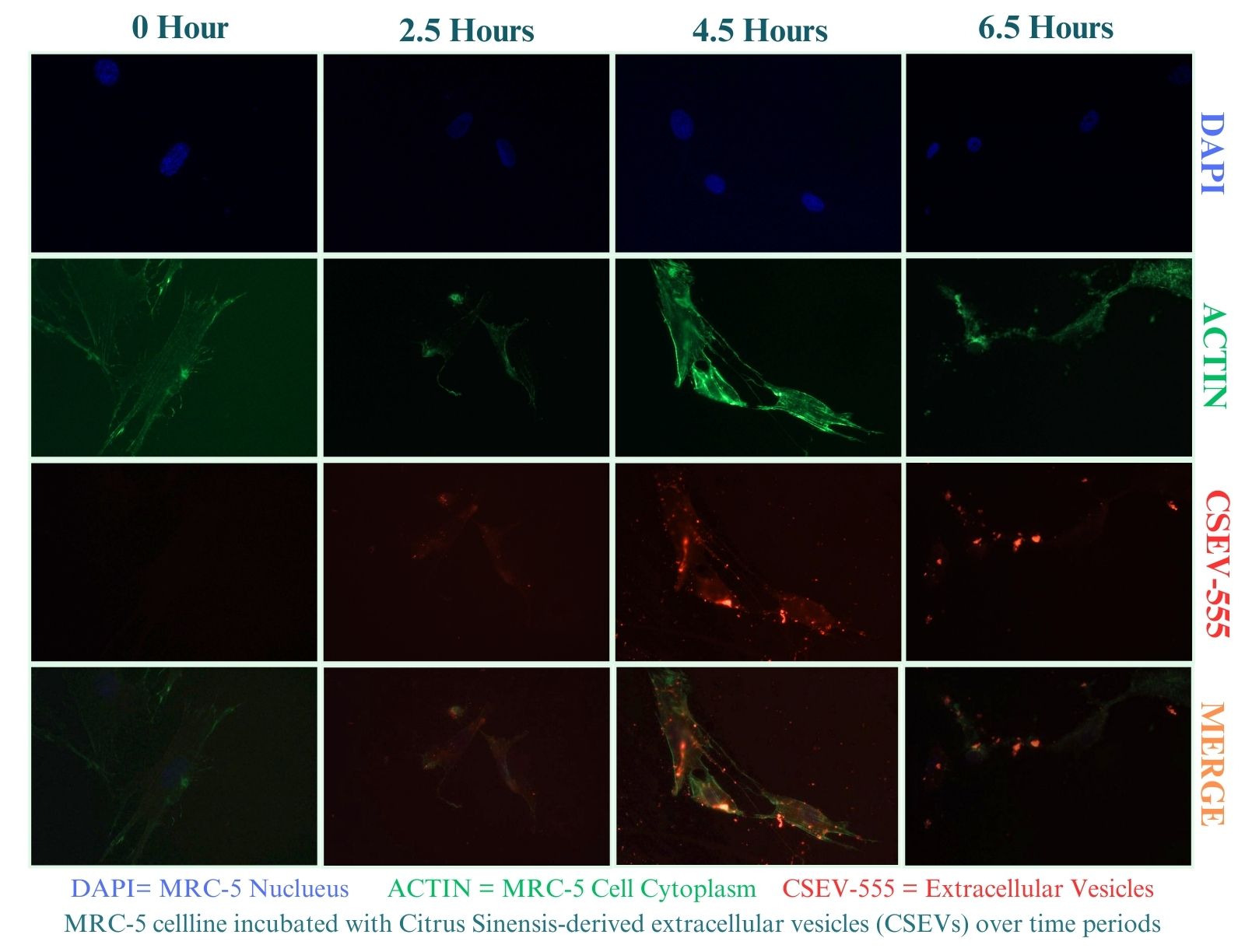
Cellular Uptake of Citrus Sinensis-Derived Extracellular Vesicles in MRC-5
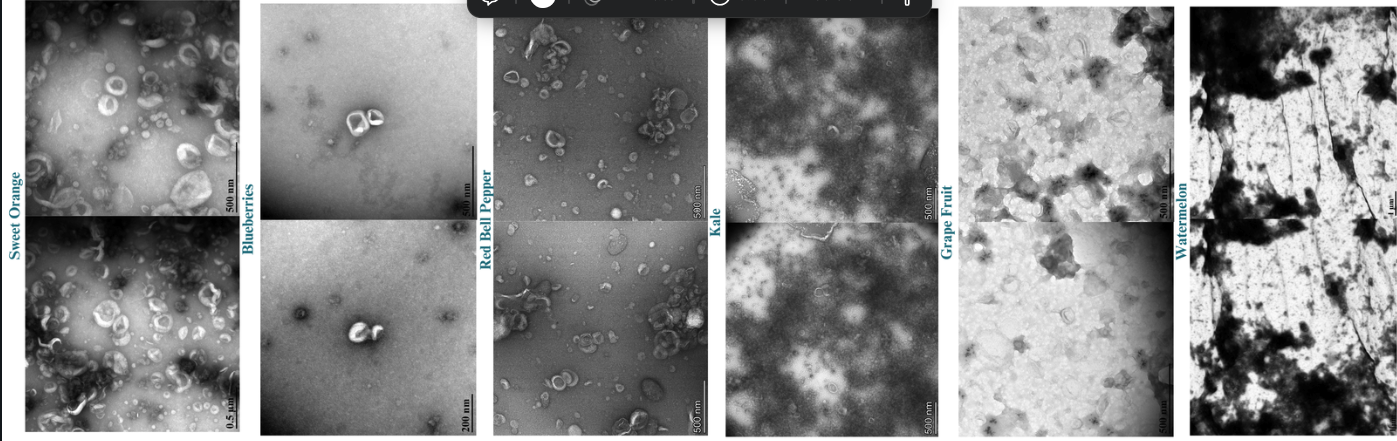
Transmission Electron Microscopy images of various plant-derived extracellular vesicles.
Methods: To assess the optimization of PDEV isolation, we developed a robust methodology for isolating extracellular vesicles from Citrus Sinensis, Citrullus lanatus, Brassica oleracea, Brassica oleracea, and Capsicum annuum via ultracentrifugation, subsequently characterizing them through transmission electron microscopy (TEM) and nanoparticle tracking analysis (NTA). To demonstrate cellular uptake of PDEVs in mammalian cells, Citrus Sinensis-derived Extracellular vesicles (CSEVs) were labeled with BioTracker 555 Orange Dye and incubated with Medical Research Council cell strain 5 (MRC-5). cells at various time points (2.5 hours, 4.5 hours, and 6.5 hours), followed by fixation and imaging using light microscopy. To demonstrate comparative drug delivery and therapeutic potential, we explored the cargo-loading abilities of Citrullus lanatusas via a microfluidic device as well as cellular uptake with mammalian cells using PDEVs.
Results: A robust methodology for isolating various plant-derived extracellular vesicles (PDEVs) via ultracentrifugation was developed and subsequently characterized through transmission electron microscopy (90-170 nm) and nanoparticle tracking analysis (1.0 x 10^9 - 1.0 x 10^11 particles/mL). Notable cellular uptake of CSEVs by MRC-5 cells across all time points, with an initial uptake percentage of 44.37% at 2.5 hrs, peaking at 50.33% by 4.5 hrs, and declining to 25.93% at 6.5 hrs. This study showcases the rapid uptake of CSEVs by MRC-5 cells within 2.5 hours, reaching optimal uptake at 4.5 hours.
Conclusion: These results underscore the potential of PDEVs as a viable drug delivery system for diverse diseases. As an alternative to mammalian EVs, PDEVs have the advantages of easy and inexpensive access, the possibility of large-scale production, and the elimination of culture systems. Future investigations should delve into comparing the drug-loading capabilities of PDEVs with both standalone and mammalian-derived EVs, shedding light on their comparative efficacy and therapeutic potential.

This Figure compares standard characterization methods for various plant-derived extracellular vesicles.

Cellular Uptake of Citrus Sinensis-Derived Extracellular Vesicles in MRC-5

Transmission Electron Microscopy images of various plant-derived extracellular vesicles.
