Preclinical, Clinical, and Translational Sciences
(M1230-05-31) Physiologically Based Biopharmaceutics Modeling of Formulation Bioequivalence for Mobocertinib, a Weak Base, Using pH-Dependent Dissolution Data
- MP
Michael E. Perlman, PhD
Associate Scientific Fellow, Drug Product Development and Devices/Analytical Development
Takeda Pharmaceutical Company Limited
Needham, Massachusetts, United States - MP
Michael E. Perlman, PhD
Associate Scientific Fellow, Drug Product Development and Devices/Analytical Development
Takeda Pharmaceutical Company Limited
Needham, Massachusetts, United States - SZ
Steven Zhang
Clinical Pharmacology Lead
Takeda Pharmaceutical Company Limited
Belmont, Massachusetts, United States - MH
Michael Hanley
Senior Director, Quantitative Clinical Pharmacology
Takeda Pharmaceutical Company Limited
Cambridge, Massachusetts, United States - JD
Jin Dong
Senior Scientist II
Simulations Plus, Inc.
Gaithersburg, Maryland, United States - TV
Tarang B. Vora
Senior Scientist II
Simulations Plus, Inc.
Research Triangle Park, North Carolina, United States - GF
Grace Fraczkiewicz
Vice President, PBPK Services
Simulations Plus, Inc.
Research Triangle Park, North Carolina, United States - ZT
Zach Thompson
Scientist
Takeda Pharmaceutical Company Limited
Wilmington, Massachusetts, United States - KM
Kazunobu Moriguchi
Director of Analytical Development
Takeda Pharmaceutical Company Limited
Cambridge, Massachusetts, United States 
Neeraj Gupta, Ph.D.
Executive Director, Quantitative Clinical Pharmacology
Takeda Pharmaceutical Company Limited
Cambridge, Massachusetts, United States- LD
Landon Durak
Senior Scientist, Synthetic Molecule Process Development
Takeda Pharmaceutical Company Limited
New Haven, Connecticut, United States - JQ
Justin Quon
Senior Staff Engineer, Synthetic Molecule Process Development
Takeda Pharmaceutical Company Limited
New Haven, Connecticut, United States - YK
Yuki Kodono
Japan Regional Product Lifecycle Management
Takeda Pharmaceutical Company Limited
Yodogawa-ku, Osaka, Japan - IA
Ian Armitage
Head, AD Early Development US
Takeda Pharmaceutical Company Limited
Cambridge, Massachusetts, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: PBBM modeling was performed with GastroPlus® version 9.7 (Simulations Plus, Inc.),with calculation of missing properties from chemical structure using ADMET Predictor®. Dissolution experiments at pH 1.2 were performed with USP apparatus II at 50 rpm in 500 mL of pH 1.2 medium, and with USP apparatus I at 100 rpm in 500 mL of pH 4.5, 6.5 and 6.8 media. pH-dependent Z-factors were calculated in GastroPlus®. Equilibrium solubilities at 37°C, pKa values, and log P/D were measured. The human effective permeability (Peff) was calculated from Caco2 data (15.0 x 10-6 cm/s). The mechanistic absorption/PBPK model was built with all perfusion-limited tissues and validated using IV and PO solution data.1 Systemic clearance was parameterized as CYP3A4 metabolism (from in vitro data: liver fm=0.74, gut fm=1), and the rest of liver CL was expressed as linear CL and was not assigned to any specific enzyme. High enterocyte binding was used to capture the delayed observed Tmax (4 – 6 hours post dose),2 and which can arise from lysosomal trapping3 of highly lipophilic bases (Log P 4.65). Virtual populations were generated to match the number and demographics of individual healthy subject data from a previous clinical trial conducted with Process A and B DiC’s (an rBA study)2 (n=25) at the 160 mg dose. Bioequivalence was assessed using 10 virtual cross-over trials and pH-dependent dissolution data.
Results: Rapid dissolution was observed for API Process A, B and C DiC’s ( >85% in 30 min) at pH 1.2, 4.5 (Figure 1A), and 6.5. However, at pH 6.8 dissolution was highly variable and not rapid for Process B and C DiC’s (Figure 1B), perhaps related to the steep decrease in solubility noted at pH 7.0. Dissolution data alone was not sufficient to support bioequivalence and therefore PBBM model development was undertaken. The PBBM model was able to adequately describe the observed plasma PK profiles for Process A and B DiC’s. When comparing the simulation results of the virtual crossover trial (fasted state, Process B as reference), with Z-factors from pH 1.2, 4.5, 6.5 and 6.8 as input, it was shown that the 90% confidence intervals of the geometric mean ratios of Cmax (86.95~108.19%), AUC0-t (83.84~115.97%), and AUC0-inf (82.89~117.47%) fell within the bioequivalence range of 80 to 125%. Virtual BE simulations using only a single Z-factor at pH 6.8 failed to show bioequivalence. Bioequivalence was predicted between Process C/size 1 and Process B/size 1 (reference) capsules (Figure 2) and between Process C/size 2 and Process C/size 1 (reference) capsules. Parameter sensitivity analysis (PSA) (Figure 3) showed that there is no impact of mean particle size up to 260 µm (Process B = 80 µm).
Conclusion: The virtual BE simulations based on pH-dependent dissolution data predicted bioequivalence of API Process A and B DiC’s in healthy fasted subjects, which is consistent with the results of a clinical rBA study2 comparing process B/size 1 and process A/size 2 DiC’s. Virtual BE simulations also predicted bioequivalence for Process B and C DiC’s. API Process C provides better particle control as a result of milling and enables the use of smaller capsules (due to higher bulk density). The PBBM results are also consistent with previously conducted population PK analyses, which indicated bioequivalence for Process A, B, and C DiC’s.4 The lack of bioequivalence shown using pH 6.8 dissolution data alone indicated that this condition is not predictive of in vivo dissolution. This result is expected since pH 6.8 is characteristic of the distal small intestine (lower ileum) and colon, while mobocertinib absorption is thought to take place mostly in the upper small intestine. Finally, the PSA results for mean particle size illustrate the lack of impact on bioavailability of milling utilized for API Process C.
References: 1. M. Hanley, S. Zhang, R. Griffin, S. Zhu, R. Fram, J. Lin, K. Venkatakrishnan, N. Gupta. A phase 1 study to assess the absolute bioavailability, mass balance, pharmacokinetics, metabolism, and excretion of [14C]-mobocertinib, an oral inhibitor of EGFR exon 20 insertion mutations, in healthy participants. Invest New Drugs 42 (2024): 343-352.
2. S. Zhang, S. Jin, C. Griffin, Z. Feng, J. Lin, M. Baratta, R. Brake, K. Venkatakrishnan, N. Gupta. Single-dose pharmacokinetics and tolerability of the oral epidermal growth factor receptor inhibitor mobocertinib (TAK-788) in healthy volunteers: low-fat meal effect and relative bioavailability of 2 capsule products. Clin Pharmacol Drug Dev 10 (2021):1028-1043.
3. M. Bolger, J. Macwan, M. Sarfraz, M. Almukainzi, R. Lobenberg. The irrelevance of in vitro dissolution in setting product specifications for drugs like dextromethorphan that are subject to lysosomal trapping. J Pharm Sci 108 (2019): 268-278.
4. N. Gupta, P. Pierillas, M. Hanley, S. Zhang, P. Diderichsen. Population pharmacokinetics of mobocertinib in healthy volunteers and patients with non-small cell lung cancer. CPT Pharmacometrics Syst Pharmacol 11(2022):731-744.
.jpg) Figure 1. Dissolution of Mobocertinib Development Capsules at (A) pH 4.5 and (B) pH 6.8.
Figure 1. Dissolution of Mobocertinib Development Capsules at (A) pH 4.5 and (B) pH 6.8. .jpg) Figure 2. Virtual Bioequivalence Assessment of TAK-788 Capsule Process C/Size 1 and Capsule Process B/Size 1 at a Dose of 160 mg, using Z-factors for pH 1.2, 4.5, 6.5 and 6.8.
Figure 2. Virtual Bioequivalence Assessment of TAK-788 Capsule Process C/Size 1 and Capsule Process B/Size 1 at a Dose of 160 mg, using Z-factors for pH 1.2, 4.5, 6.5 and 6.8.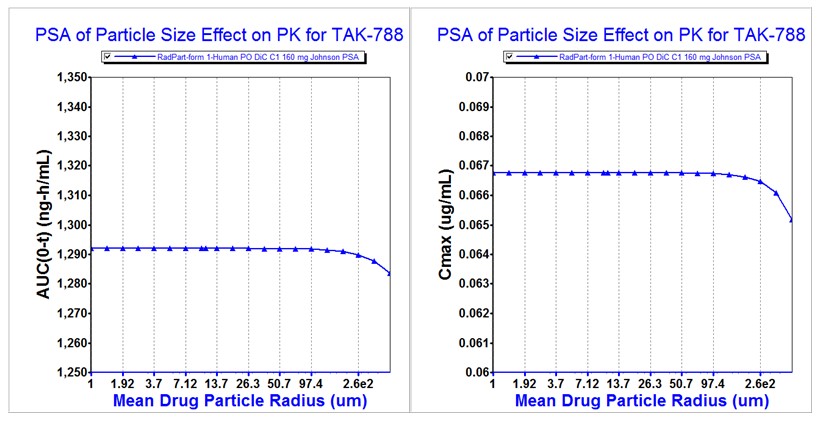 Figure 3. Sensitivity of Capsule Process C/Size 1 Mobocertinib PK Parameters to the Variation of Particle Size between 1 and 500 μm (SD= 0).
Figure 3. Sensitivity of Capsule Process C/Size 1 Mobocertinib PK Parameters to the Variation of Particle Size between 1 and 500 μm (SD= 0). 