Formulation and Delivery - Biomolecular
(M1230-08-49) High-Concentration Protein Formulations: Mechanistic Understanding of Hydroxypropyl-β-Cyclodextrins Mitigates Viscosity Challenges
Monday, November 10, 2025
12:30 PM - 1:30 PM CT

TAO PENG, PhD
Senior BioPharma Research Manager
Roquette Asia Pacific Pte Ltd
Singapore, Singapore- JH
JIAYI HUANG, Ph.D.
Biopharma Scientist
Roquette Asia Pacific Pte. Ltd.
Singapore, Singapore 
SHIQI HONG, PhD (she/her/hers)
Biopharma Team Lead
Roquette Asia Pacific Pte. Ltd.
Singapore, Singapore
Lucas Goh, PhD (he/him/his)
Biopharma Team Lead
Roquette Asia Pacific Pte Ltd
Singapore, Singapore- KC
KEAT-THENG CHOW, Ph.D.
Head of Applied Sciences Pharma
Roquette Asia Pacific Pte. Ltd.
Singapore, Singapore 
Deepak Bahl, PhD
Global Head of Applied Sciences - Pharma
Roquette
Lower Gwynedd Township, Pennsylvania, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Protein therapeutics are vital for treating a wide range of diseases due to their high specificity and efficacy. Subcutaneous (SC) delivery is preferred for its convenience and cost-effectiveness but requires highly concentrated formulations, leading to elevated viscosity from protein–protein interactions.1, 2 This hampers manufacturing, injection, and patient compliance.1 The limited effectiveness of conventional excipients in reducing viscosity highlights the need for alternatives that can more reliably enable the formulation and delivery of high-concentration protein therapeutics. This study highlights the innovative role of hydroxypropyl β-cyclodextrin (HPβCD) as a viscosity-reducing excipient and investigates its underlying mechanism of action.
Methods: Hydroxypropyl beta-cyclodextrin (KLEPTOSE® HPB Biopharma) was obtained from Roquette Frères (Lestrem, France). All other research-grade chemicals were obtained from Merck KGaA (Darmstadt, Germany) and are multi-compendia or USP grade. Ipilimumab was produced in Chinese hamster ovary (CHO) cells and purified in-house. Cadonilimab and ramucirumab were obtained from A*STAR Bioprocessing Technology Institute (BTI) of Singapore. Infliximab was obtained from BOC Sciences (Shirley, NY, USA). Ipilimumab was prepared in buffer containing 5.85 mg/mL sodium chloride (NaCl) and 3.15 mg/mL Tris-hydrochloride, adjusted to pH 7.0, and concentrated to 200 mg/mL. Ipilimumab was further formulated at 100 to 180 mg/mL, with the addition of 0.5% - 20% KLEPTOSE® HPB. Other excipients, including arginine, lysine, histidine, proline, mannitol, sorbitol, sucrose, or polysorbate 80 at different concentrations, were formulated with ipilimumab at 180 mg/mL for comparison. Viscosity measurements for all formulations were conducted using a microVISC Viscometer (RheoSense, San Ramon, CA), with a shear rate of 550 s-1 at 25 °C. Triplicate runs were conducted for each formulation, and the average viscosity reading and standard deviation were calculated. Diffusion interaction parameter KD and second virial coefficient B22 of mAbs were determined using Stunner (Unchained Labs, Pleasanton, CA).
Results: Preliminary evaluation of three high-viscosity monoclonal antibodies (mAbs) at high concentrations, including ipilimumab, infliximab, and faricimab, had demonstrated very effective viscosity reduction by HPβCD. Ipilimumab was selected as the model high-viscosity protein for detailed studies. Our results demonstrated that incorporating HPβCD ranging 0.5 – 20% (w/v) significantly reduced the viscosity of ipilimumab formulations at concentration of 100 – 180 mg/mL, achieving levels well below the syringeability threshold. A 5% (w/v) concentration of HPβCD was found to be the most effective, especially in formulations containing 180 mg/mL ipilimumab, where it reduced the viscosity from 39.5 mPa·s to 16.5 mPa·s (Figure 1). Compared to other commonly used viscosity-reducing excipients, the high-concentration ipilimumab formulation (180 – 190 mg/mL) containing 5% HPβCD exhibited the lowest viscosity (Figure 2). While the addition of arginine or proline alone can effectively reduce ipilimumab's viscosity from 60 mPa·s to a range of 33-50 mPa·s, the inclusion of 5% HPβCD further decreased the viscosity to 18 mPa·s. Furthermore, combinations of other excipients with HPβCD did not provide further viscosity reduction, indicating that the addition of 5% HPβCD alone was sufficient to provide the best viscosity reduction effect. HPβCD also effectively modulates the osmolality of the formulation. Unlike other viscosity-reducing excipients, which significantly increase the osmolality of ipilimumab formulations at 180 mg/mL, the addition of up to 10% HPβCD resulted in only a modest increase of approximately 100 mOsm/kg. This leaves sufficient room for incorporating additional excipients to optimize the formulation for subcutaneous administration. Mechanistic insights obtained through the evaluation of diffusion interaction parameter (kD) and the second viral coefficient (B22) revealed that HPβCD disrupts attractive intermolecular interactions between protein molecules, thereby reducing viscosity. The kD value of ipilimumab decreased from -12.08 mL/g in the absence of HPβCD to -7.316 mL/g in its presence (Figure 3A). Similarly, the B22 value shifted from -3.33 × 10⁻⁵ mL·mol/g² without HPβCD to 4.36 × 10⁻⁵ mL·mol/g² with HPβCD (Figure 3B). The increase in B22 from a negative to a positive value upon addition of HPβCD suggests that KLEPTOSE® HPB reduces the attractive interactions between ipilimumab molecules, consistent with the observed changes in kD. The weakened attractive interactions accounts for the reduced viscosity of ipilimumab. Similar trend were observed in another model mAb - infliximab (Figure 3). In contrast, other mAbs, such as cadonilimab and ramucirumab exhibited positive KD and B22 values even in the absence of HPβCD, indicating inherently repulsive intermolecular forces and a corresponding lower risk of high viscosity. These parameters can serve as a tool to identify proteins prone to high viscosity and to assess the potential of HPβCD to reduce protein–protein interactions and lower viscosity.
Conclusion: As a well-established excipient in many approved parenteral formulations, HPβCD emerges as a promising solution for high-concentration protein formulations. By significantly enhancing injectability, stability, and manufacturability, HPβCD addresses key industry challenges, paving the way for patient-friendly, high-dose subcutaneous delivery.
References: 1. Banik, N.; Braun, S.; Brandenburg, J. G.; Fricker, G.; Kalonia, D. S.; Rosenkranz, T., Technology development to evaluate the effectiveness of viscosity reducing excipients. International Journal of Pharmaceutics 2022, 626, 122204.
2. Prašnikar, M.; Žiberna, M. B.; Kržišnik, N.; Roškar, R.; Grabnar, I.; Žula, A.; Grabnar, P. A., Additive effects of the new viscosity-reducing and stabilizing excipients for monoclonal antibody formulation. International journal of pharmaceutics 2025, 674, 125451.
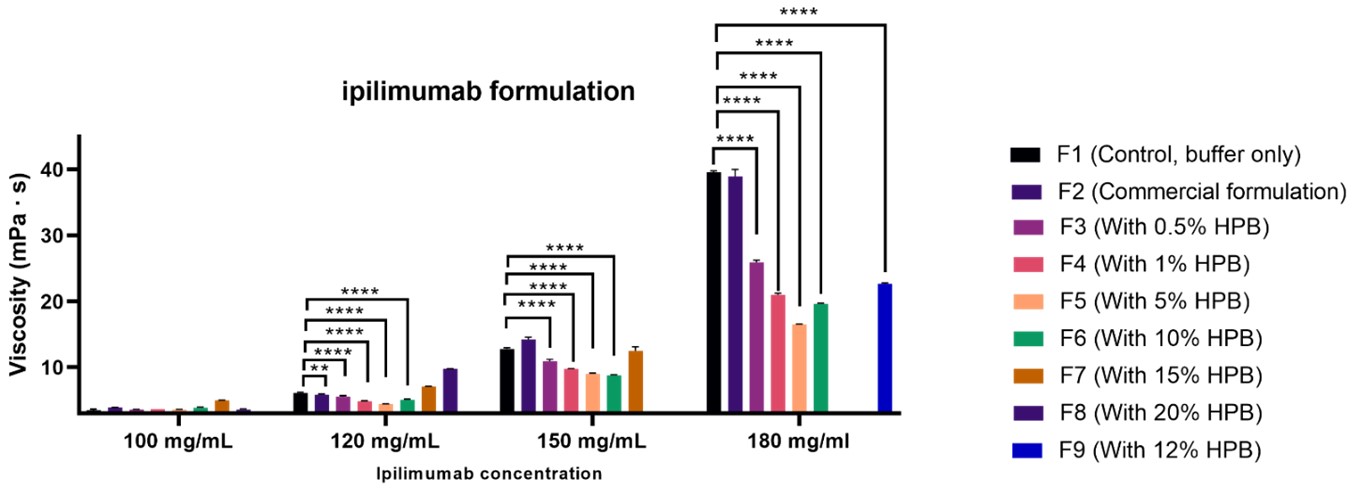 Viscosities of formulations with different concentrations of ipilimumab and KLEPTOSE® HPB. ** p < 0.01, *** p < 0.001, and **** p < 0.0001 using one-way ANOVA compared to the Control.
Viscosities of formulations with different concentrations of ipilimumab and KLEPTOSE® HPB. ** p < 0.01, *** p < 0.001, and **** p < 0.0001 using one-way ANOVA compared to the Control.
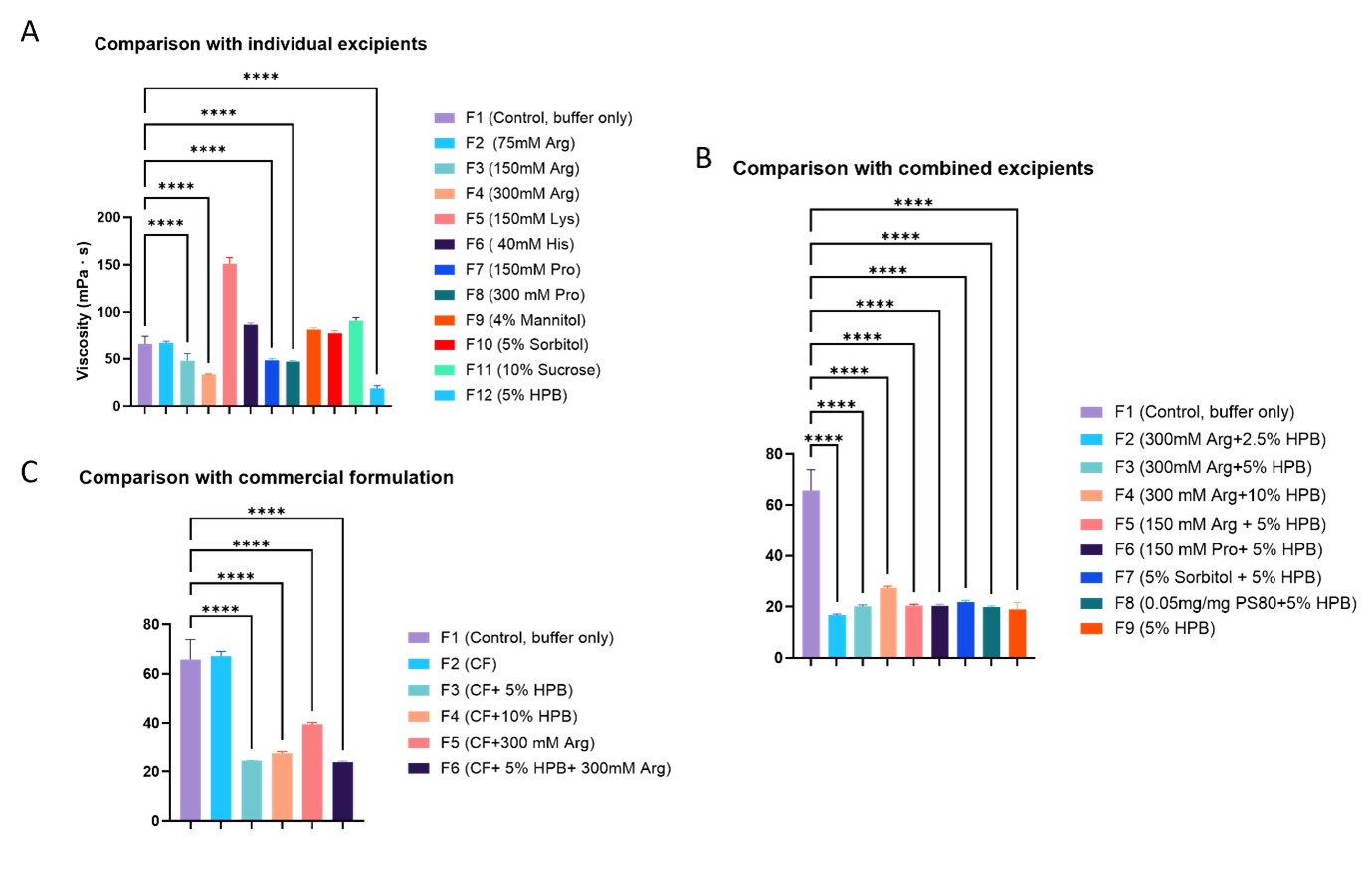 Viscosities of ipilimumab formulations containing different excipients at varying concentrations. Comparison with individual excipients (A), combined excipients (B) and within commercial formulation of ipilimumab (designed for low concentration formulation) (C). **** p < 0.0001 using one-way ANOVA compared to the control.
Viscosities of ipilimumab formulations containing different excipients at varying concentrations. Comparison with individual excipients (A), combined excipients (B) and within commercial formulation of ipilimumab (designed for low concentration formulation) (C). **** p < 0.0001 using one-way ANOVA compared to the control.
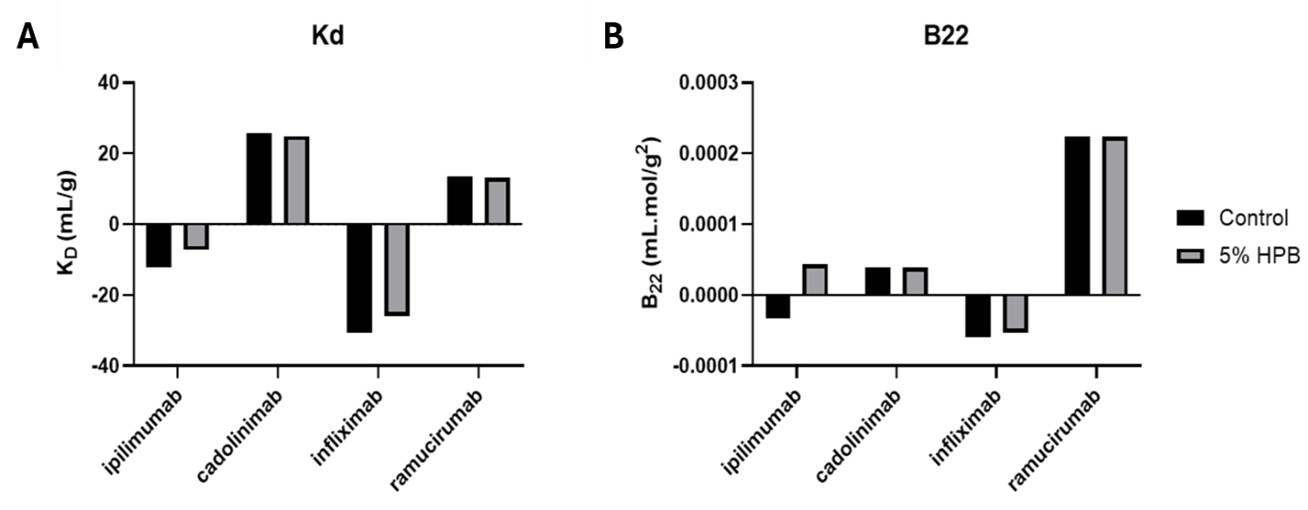 KD and B22 values of formulations containing ipilimumab, cadonilimab, infliximab, and ramucirumab, in the presence and absence of KLEPTOSE® HPB.
KD and B22 values of formulations containing ipilimumab, cadonilimab, infliximab, and ramucirumab, in the presence and absence of KLEPTOSE® HPB.
Methods: Hydroxypropyl beta-cyclodextrin (KLEPTOSE® HPB Biopharma) was obtained from Roquette Frères (Lestrem, France). All other research-grade chemicals were obtained from Merck KGaA (Darmstadt, Germany) and are multi-compendia or USP grade. Ipilimumab was produced in Chinese hamster ovary (CHO) cells and purified in-house. Cadonilimab and ramucirumab were obtained from A*STAR Bioprocessing Technology Institute (BTI) of Singapore. Infliximab was obtained from BOC Sciences (Shirley, NY, USA). Ipilimumab was prepared in buffer containing 5.85 mg/mL sodium chloride (NaCl) and 3.15 mg/mL Tris-hydrochloride, adjusted to pH 7.0, and concentrated to 200 mg/mL. Ipilimumab was further formulated at 100 to 180 mg/mL, with the addition of 0.5% - 20% KLEPTOSE® HPB. Other excipients, including arginine, lysine, histidine, proline, mannitol, sorbitol, sucrose, or polysorbate 80 at different concentrations, were formulated with ipilimumab at 180 mg/mL for comparison. Viscosity measurements for all formulations were conducted using a microVISC Viscometer (RheoSense, San Ramon, CA), with a shear rate of 550 s-1 at 25 °C. Triplicate runs were conducted for each formulation, and the average viscosity reading and standard deviation were calculated. Diffusion interaction parameter KD and second virial coefficient B22 of mAbs were determined using Stunner (Unchained Labs, Pleasanton, CA).
Results: Preliminary evaluation of three high-viscosity monoclonal antibodies (mAbs) at high concentrations, including ipilimumab, infliximab, and faricimab, had demonstrated very effective viscosity reduction by HPβCD. Ipilimumab was selected as the model high-viscosity protein for detailed studies. Our results demonstrated that incorporating HPβCD ranging 0.5 – 20% (w/v) significantly reduced the viscosity of ipilimumab formulations at concentration of 100 – 180 mg/mL, achieving levels well below the syringeability threshold. A 5% (w/v) concentration of HPβCD was found to be the most effective, especially in formulations containing 180 mg/mL ipilimumab, where it reduced the viscosity from 39.5 mPa·s to 16.5 mPa·s (Figure 1). Compared to other commonly used viscosity-reducing excipients, the high-concentration ipilimumab formulation (180 – 190 mg/mL) containing 5% HPβCD exhibited the lowest viscosity (Figure 2). While the addition of arginine or proline alone can effectively reduce ipilimumab's viscosity from 60 mPa·s to a range of 33-50 mPa·s, the inclusion of 5% HPβCD further decreased the viscosity to 18 mPa·s. Furthermore, combinations of other excipients with HPβCD did not provide further viscosity reduction, indicating that the addition of 5% HPβCD alone was sufficient to provide the best viscosity reduction effect. HPβCD also effectively modulates the osmolality of the formulation. Unlike other viscosity-reducing excipients, which significantly increase the osmolality of ipilimumab formulations at 180 mg/mL, the addition of up to 10% HPβCD resulted in only a modest increase of approximately 100 mOsm/kg. This leaves sufficient room for incorporating additional excipients to optimize the formulation for subcutaneous administration. Mechanistic insights obtained through the evaluation of diffusion interaction parameter (kD) and the second viral coefficient (B22) revealed that HPβCD disrupts attractive intermolecular interactions between protein molecules, thereby reducing viscosity. The kD value of ipilimumab decreased from -12.08 mL/g in the absence of HPβCD to -7.316 mL/g in its presence (Figure 3A). Similarly, the B22 value shifted from -3.33 × 10⁻⁵ mL·mol/g² without HPβCD to 4.36 × 10⁻⁵ mL·mol/g² with HPβCD (Figure 3B). The increase in B22 from a negative to a positive value upon addition of HPβCD suggests that KLEPTOSE® HPB reduces the attractive interactions between ipilimumab molecules, consistent with the observed changes in kD. The weakened attractive interactions accounts for the reduced viscosity of ipilimumab. Similar trend were observed in another model mAb - infliximab (Figure 3). In contrast, other mAbs, such as cadonilimab and ramucirumab exhibited positive KD and B22 values even in the absence of HPβCD, indicating inherently repulsive intermolecular forces and a corresponding lower risk of high viscosity. These parameters can serve as a tool to identify proteins prone to high viscosity and to assess the potential of HPβCD to reduce protein–protein interactions and lower viscosity.
Conclusion: As a well-established excipient in many approved parenteral formulations, HPβCD emerges as a promising solution for high-concentration protein formulations. By significantly enhancing injectability, stability, and manufacturability, HPβCD addresses key industry challenges, paving the way for patient-friendly, high-dose subcutaneous delivery.
References: 1. Banik, N.; Braun, S.; Brandenburg, J. G.; Fricker, G.; Kalonia, D. S.; Rosenkranz, T., Technology development to evaluate the effectiveness of viscosity reducing excipients. International Journal of Pharmaceutics 2022, 626, 122204.
2. Prašnikar, M.; Žiberna, M. B.; Kržišnik, N.; Roškar, R.; Grabnar, I.; Žula, A.; Grabnar, P. A., Additive effects of the new viscosity-reducing and stabilizing excipients for monoclonal antibody formulation. International journal of pharmaceutics 2025, 674, 125451.
 Viscosities of formulations with different concentrations of ipilimumab and KLEPTOSE® HPB. ** p < 0.01, *** p < 0.001, and **** p < 0.0001 using one-way ANOVA compared to the Control.
Viscosities of formulations with different concentrations of ipilimumab and KLEPTOSE® HPB. ** p < 0.01, *** p < 0.001, and **** p < 0.0001 using one-way ANOVA compared to the Control. Viscosities of ipilimumab formulations containing different excipients at varying concentrations. Comparison with individual excipients (A), combined excipients (B) and within commercial formulation of ipilimumab (designed for low concentration formulation) (C). **** p < 0.0001 using one-way ANOVA compared to the control.
Viscosities of ipilimumab formulations containing different excipients at varying concentrations. Comparison with individual excipients (A), combined excipients (B) and within commercial formulation of ipilimumab (designed for low concentration formulation) (C). **** p < 0.0001 using one-way ANOVA compared to the control. KD and B22 values of formulations containing ipilimumab, cadonilimab, infliximab, and ramucirumab, in the presence and absence of KLEPTOSE® HPB.
KD and B22 values of formulations containing ipilimumab, cadonilimab, infliximab, and ramucirumab, in the presence and absence of KLEPTOSE® HPB. 