Bioanalytics - Biomolecular
(T1030-01-01) Feasibility of Adding Volumetric Absorptive Microsampling (VAMS®) Using Mitra® Tips to an Established ELISA Assay to Determine Concentrations of a Monoclonal Antibody in Whole Blood
Tuesday, November 11, 2025
10:30 AM - 11:30 AM CT
- AB
Alexa Boyle, MS
Lead Scientist
Labcorp
Chantilly, Virginia, United States - AB
Alexa Boyle, MS
Lead Scientist
Labcorp
Chantilly, Virginia, United States - AG
Angelika Grinshpun
Method Development Scientist
Labcorp
Chantilly, Virginia, United States - SE
Sarah Eakhurst
Method Development Scientist
Labcorp
Harrogate, England, United Kingdom
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: The purpose of these experiments was to take an already existing ligand-binding ELISA assay used for determining concentrations of a monoclonal antibody in human serum and add volumetric absorptive microsampling (VAMS®) to determine concentrations of the same monoclonal antibody in human whole blood. The driving factor behind this attempt was two-fold. The first reason was that the patient population for the monoclonal antibody was moving into pediatrics, which historically has generated smaller sample volumes for pharmacokinetic (PK) testing. The use of Mitra® tips allows smaller blood volumes to be collected and utilized for PK analysis. Secondly, the use of VAMS® instead of venous blood sampling provides a more patient centric approach allowing the patient to have samples collected at the location most convenient for them, as well as, a less invasive collection.
Methods: The original ELISA assay for detection of a monoclonal antibody in human serum involves washing then adding a biotinylated conjugate to Streptavidin coated and pre-blocked 96-well plates. During the biotin conjugate incubation, the monoclonal antibody calibrators and quality controls are freshly prepared or thawed from frozen and diluted 1/100 (minimum required dilution-MRD) using sample diluent. Any additional samples are also diluted 1/100 using sample diluent. The plate is washed, and calibrators, controls and samples are added. Following the appropriate sample incubation time, the plates are washed and a HRP conjugate is added. After appropriate HRP incubation, a final wash is performed and TMB substate is added to develop color. Plates are pre-read at the appropriate wavelength and once a pre-established OD value is reached for the top calibrator, the reaction is quenched with 1M phosphoric acid. Plates are read at the appropriate wavelength, and the data is regressed using a four parameter logistic curve fitting with 1/y2 weighting. The VAMS® workflow was added to the assay for the sample pre-treatment. The appropriate volume of sample, depending on tip volume, is added to the wells of a dilution plate in order of plate layout. Using the Mitra® tips, blood is absorbed from the plate well and the tip is inserted into a labeled tube for drying. Tips are dried for 1 hour then elution buffer is added to each tube and incubation with agitation occurs. After appropriate incubation, the sample is further diluted using sample diluent to reach the 1/100 MRD. The samples are added to the pre-blocked streptavidin coated plates after biotin conjugate incubation and the remaining assay steps are followed.
Results: To determine feasibility of incorporating human whole blood samples into an already validated human serum method, the anti-coagulant required for the whole blood samples and the hematocrit (Hct) levels had to be assessed for interference. Quality controls prepared in K2EDTA plasma, 30% Hct and 50% Hct whole blood assessed against the calibration curve and quality controls prepared in qualified human serum pool showed that quality controls prepared in serum, K2EDTA plasma and whole blood on VAMS® tips are comparable. Next, accuracy and precision were tested for the VAMS® tips under different drying times and elution buffers. The results showed that the elution buffer most similar to the sample diluent yielded the best results. In addition, the accuracy and precision of the quality controls were shown to not be affected by the longer drying times resulting in the shortest drying time used for the remainder of the experiments. Following optimization, a small cohort was assessed for selectivity and one curve of dilutional linearity. All three whole blood samples spiked at the LLOQ and ran blank passed the selectivity assessment, while the dilutional linearity was able to be achieved down to a concentration between the LQC and LLOQ with the LLOQ level failing. Next, stability assessments were conducted for the eluted sample and passed at the LQC and HQC for 3 freeze-thaws, overnight at 4°C and up to 24 hours of benchtop. Stability assessments were further conducted on the dried tips and passed at the LQC and HQC for up to 7 days at -50°C, -10°C, 4°C and 25°C. Additional stability assessments were performed to allow greater than 7 days of stability for the dried tips, however no additional stability was achieved due to LQC failures.
Conclusion: The feasibility of adding the VAMS® workflow into an already established ELISA method for detecting a monoclonal antibody in human serum was not able to be achieved due to inability to establish stability greater than 7 days for dried tips. Dried tip stability for 7 days is unlikely to allow enough time for the sample to move from patient collection to testing. Future ligand-binding PK assays with the desire to incorporate a VAMS® workflow would benefit from a new method where the need was known from initiation. This knowledge would allow the assay to be developed with non-interfering buffers and a sensitivity level that matched the confines of the whole blood samples.
Acknowledgements: Neoteryx® is the supplier for the Mitra® tips utilized for the VAMS® workflow.
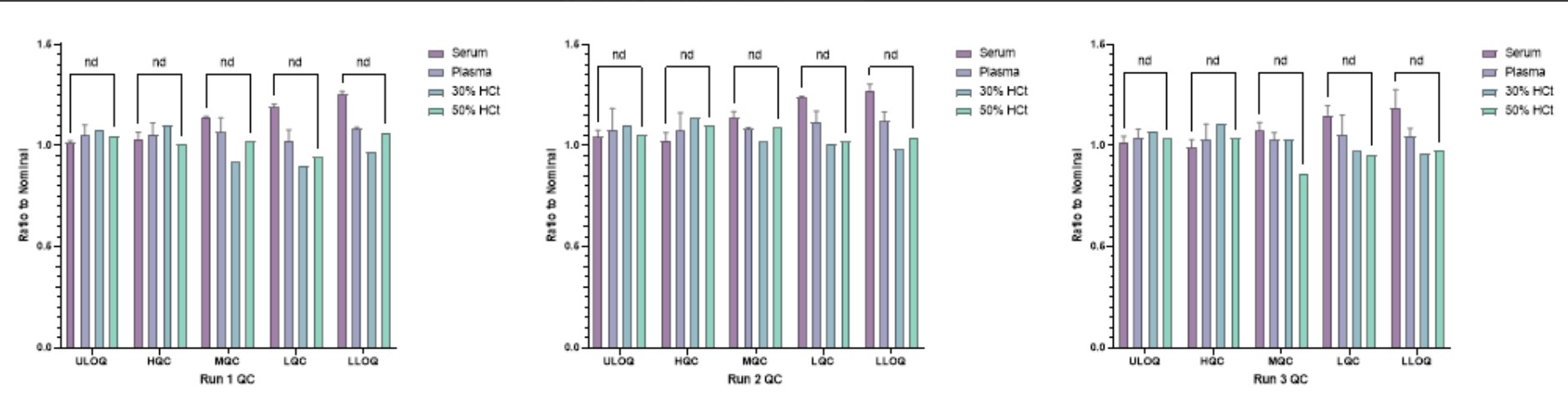 Figure 1: Comparability shown between 5 quality controls (concentrations 90 ng/mL (LLOQ), 150 ng/mL (LQC), 800 ng/mL (MQC), 2200 ng/mL (HQC) and 3000 ng/mL (ULOQ)) prepared in human serum, K2EDTA human plasma and human whole blood (30 and 50% Hct) using Mitra® tips and assessed against a human serum calibration curve. The ratio to normal was calculated for each QC level against the human serum QC result across all 3 runs. An unpaired t-test was used to calculate significant difference for each matrix type and performed independently per run. Abbreviations: nd = no difference
Figure 1: Comparability shown between 5 quality controls (concentrations 90 ng/mL (LLOQ), 150 ng/mL (LQC), 800 ng/mL (MQC), 2200 ng/mL (HQC) and 3000 ng/mL (ULOQ)) prepared in human serum, K2EDTA human plasma and human whole blood (30 and 50% Hct) using Mitra® tips and assessed against a human serum calibration curve. The ratio to normal was calculated for each QC level against the human serum QC result across all 3 runs. An unpaired t-test was used to calculate significant difference for each matrix type and performed independently per run. Abbreviations: nd = no difference
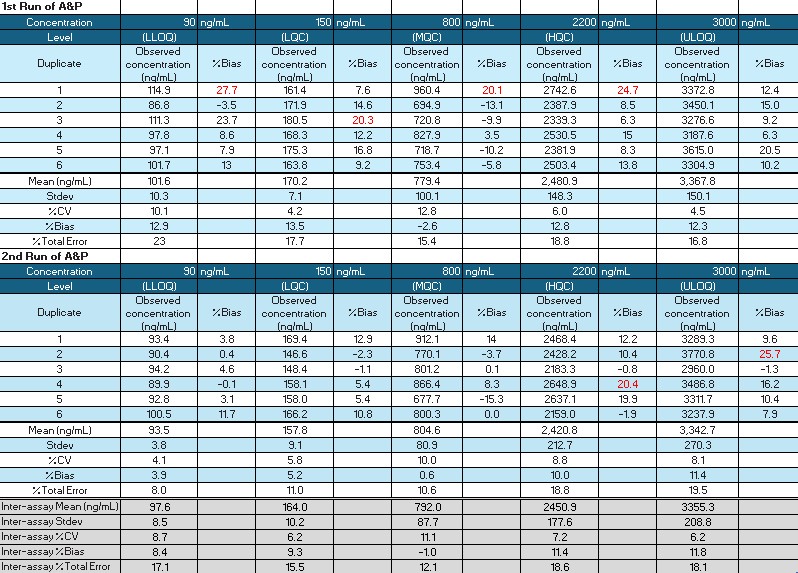 Table 1: Two runs of accuracy and precision were performed under the optimized elution buffer and drying time conditions for the 5 quality controls (concentrations 90, 150, 800, 2200 and 3000 ng/mL) prepared in whole blood using Mitra® tips. The red text for % Bias indicates that the parameter is outside the acceptance criteria: LLOQ and ULOQ < +25%, LQC/MQC/HQC < +20%.
Table 1: Two runs of accuracy and precision were performed under the optimized elution buffer and drying time conditions for the 5 quality controls (concentrations 90, 150, 800, 2200 and 3000 ng/mL) prepared in whole blood using Mitra® tips. The red text for % Bias indicates that the parameter is outside the acceptance criteria: LLOQ and ULOQ < +25%, LQC/MQC/HQC < +20%.
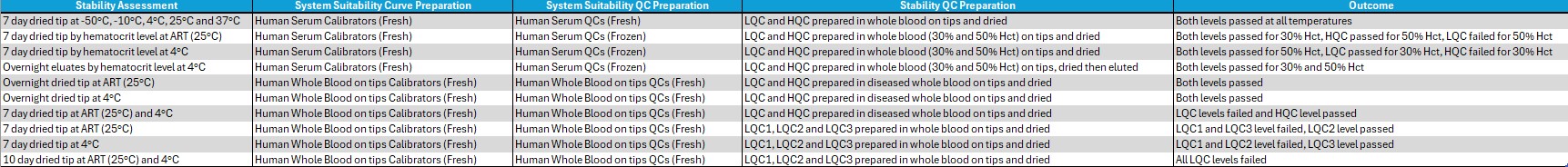 Table 2: Summary table of stability assessments performed with outcome.
Table 2: Summary table of stability assessments performed with outcome.
Methods: The original ELISA assay for detection of a monoclonal antibody in human serum involves washing then adding a biotinylated conjugate to Streptavidin coated and pre-blocked 96-well plates. During the biotin conjugate incubation, the monoclonal antibody calibrators and quality controls are freshly prepared or thawed from frozen and diluted 1/100 (minimum required dilution-MRD) using sample diluent. Any additional samples are also diluted 1/100 using sample diluent. The plate is washed, and calibrators, controls and samples are added. Following the appropriate sample incubation time, the plates are washed and a HRP conjugate is added. After appropriate HRP incubation, a final wash is performed and TMB substate is added to develop color. Plates are pre-read at the appropriate wavelength and once a pre-established OD value is reached for the top calibrator, the reaction is quenched with 1M phosphoric acid. Plates are read at the appropriate wavelength, and the data is regressed using a four parameter logistic curve fitting with 1/y2 weighting. The VAMS® workflow was added to the assay for the sample pre-treatment. The appropriate volume of sample, depending on tip volume, is added to the wells of a dilution plate in order of plate layout. Using the Mitra® tips, blood is absorbed from the plate well and the tip is inserted into a labeled tube for drying. Tips are dried for 1 hour then elution buffer is added to each tube and incubation with agitation occurs. After appropriate incubation, the sample is further diluted using sample diluent to reach the 1/100 MRD. The samples are added to the pre-blocked streptavidin coated plates after biotin conjugate incubation and the remaining assay steps are followed.
Results: To determine feasibility of incorporating human whole blood samples into an already validated human serum method, the anti-coagulant required for the whole blood samples and the hematocrit (Hct) levels had to be assessed for interference. Quality controls prepared in K2EDTA plasma, 30% Hct and 50% Hct whole blood assessed against the calibration curve and quality controls prepared in qualified human serum pool showed that quality controls prepared in serum, K2EDTA plasma and whole blood on VAMS® tips are comparable. Next, accuracy and precision were tested for the VAMS® tips under different drying times and elution buffers. The results showed that the elution buffer most similar to the sample diluent yielded the best results. In addition, the accuracy and precision of the quality controls were shown to not be affected by the longer drying times resulting in the shortest drying time used for the remainder of the experiments. Following optimization, a small cohort was assessed for selectivity and one curve of dilutional linearity. All three whole blood samples spiked at the LLOQ and ran blank passed the selectivity assessment, while the dilutional linearity was able to be achieved down to a concentration between the LQC and LLOQ with the LLOQ level failing. Next, stability assessments were conducted for the eluted sample and passed at the LQC and HQC for 3 freeze-thaws, overnight at 4°C and up to 24 hours of benchtop. Stability assessments were further conducted on the dried tips and passed at the LQC and HQC for up to 7 days at -50°C, -10°C, 4°C and 25°C. Additional stability assessments were performed to allow greater than 7 days of stability for the dried tips, however no additional stability was achieved due to LQC failures.
Conclusion: The feasibility of adding the VAMS® workflow into an already established ELISA method for detecting a monoclonal antibody in human serum was not able to be achieved due to inability to establish stability greater than 7 days for dried tips. Dried tip stability for 7 days is unlikely to allow enough time for the sample to move from patient collection to testing. Future ligand-binding PK assays with the desire to incorporate a VAMS® workflow would benefit from a new method where the need was known from initiation. This knowledge would allow the assay to be developed with non-interfering buffers and a sensitivity level that matched the confines of the whole blood samples.
Acknowledgements: Neoteryx® is the supplier for the Mitra® tips utilized for the VAMS® workflow.
 Figure 1: Comparability shown between 5 quality controls (concentrations 90 ng/mL (LLOQ), 150 ng/mL (LQC), 800 ng/mL (MQC), 2200 ng/mL (HQC) and 3000 ng/mL (ULOQ)) prepared in human serum, K2EDTA human plasma and human whole blood (30 and 50% Hct) using Mitra® tips and assessed against a human serum calibration curve. The ratio to normal was calculated for each QC level against the human serum QC result across all 3 runs. An unpaired t-test was used to calculate significant difference for each matrix type and performed independently per run. Abbreviations: nd = no difference
Figure 1: Comparability shown between 5 quality controls (concentrations 90 ng/mL (LLOQ), 150 ng/mL (LQC), 800 ng/mL (MQC), 2200 ng/mL (HQC) and 3000 ng/mL (ULOQ)) prepared in human serum, K2EDTA human plasma and human whole blood (30 and 50% Hct) using Mitra® tips and assessed against a human serum calibration curve. The ratio to normal was calculated for each QC level against the human serum QC result across all 3 runs. An unpaired t-test was used to calculate significant difference for each matrix type and performed independently per run. Abbreviations: nd = no difference Table 1: Two runs of accuracy and precision were performed under the optimized elution buffer and drying time conditions for the 5 quality controls (concentrations 90, 150, 800, 2200 and 3000 ng/mL) prepared in whole blood using Mitra® tips. The red text for % Bias indicates that the parameter is outside the acceptance criteria: LLOQ and ULOQ < +25%, LQC/MQC/HQC < +20%.
Table 1: Two runs of accuracy and precision were performed under the optimized elution buffer and drying time conditions for the 5 quality controls (concentrations 90, 150, 800, 2200 and 3000 ng/mL) prepared in whole blood using Mitra® tips. The red text for % Bias indicates that the parameter is outside the acceptance criteria: LLOQ and ULOQ < +25%, LQC/MQC/HQC < +20%. Table 2: Summary table of stability assessments performed with outcome.
Table 2: Summary table of stability assessments performed with outcome.