Formulation and Delivery - Chemical
(T1030-09-61) Quantitative Differences in Carbomer Homopolymer Type B and Its Effect on the Structural and Permeation Characteristics of Diclofenac Sodium Topical Gels

Nethra Viswaroopan, BS
Ph.D. Student
Mercer University
Atlanta, Georgia, United States- NV
Nethra Viswaroopan, BS
PhD student
Mercer University
Atlanta, Georgia, United States 
Anuradha Dey, MS (she/her/hers)
Graduate PhD Student
Mercer University
Atlanta, Georgia, United States
Meheli Ghosh, MS
Graduate Student
Mercer University
Atlanta, Georgia, United States- AR
Ariana Radmard, Pharm.D.
Ph.D. Student
Mercer University
Atlanta, Georgia, United States - MN
Mengmeng Niu, Ph.D.
Senior Pharmacologist
US Food and Drug Administration
Maryland, Maryland, United States 
Priyanka Ghosh, Ph.D.
Lead Pharmacologist at Office of Research and Standards
US Food and Drug Administration
Maryland, Maryland, United States
Ajay K. K. Banga, PhD
Professor
Mercer University
Atlanta, Georgia, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: Five diclofenac sodium gels (0.5% w/w) were prepared using Carbopol® 974P at concentrations representing ±10% (G3, G4) and ±25%(G2,G5) difference from a reference concentration (G1) (0.5% w/w)(Table 1). Gels were prepared with optimized processing parameters (Figure 1). Each formulation was evaluated (n=3) for visual appearance, pH over 7 days, specific gravity, rheology (amplitude sweep, frequency sweep, and flow curves), and texture analysis (texture profile analysis). A finite dose IVPT study was conducted using human cadaver skin (3 donors, 4 replicates per donor) in Franz diffusion cells. Receptor solution was selected based on solubility of diclofenac sodium in phosphate buffer saline ensuring the maintenance of sink conditions. Receptor sampling was conducted at predetermined time-points for diclofenac sodium quantification using high-performance liquid chromatography (HPLC).
Results: All formulations remained clear and physically stable with minimal pH variation over 7 days and a net pH change of less than 0.15. Specific gravity values remained consistent across formulations, indicating that concentration differences in Carbopol® 974P did not significantly alter gel density or internal air entrapment (Figure 2). Rheological analysis showed a Carbopol® 974P concentration-dependent increase in viscosity and yield stress. At low shear rate (2s-1), viscosity (Pa.s) was as follows: G1: 6.39 ± 0.22, G2: 2.26 ± 0.86, G3: 3.25 ± 0.01, G4: 11.27 ± 0.48, G5: 9.68 ± 0.43. Yield Stress ranged from lowest being 2.73 ± 1.14 (G2) to highest being 15.59 ± 0.91 (G4). G2 – G4 exhibited higher yield stress than G5 (0.625%), suggesting that very high Carbopol® 974P concentrations may reduce polymer mobility. Frequency sweep results demonstrated that storage modulus (G′) increased with Carbopol® 974P concentration (e.g., G′ at 1 rad/s: G2:19.13 ± 0.39, G5: 172.6 ± 2.86 Pa), reflecting enhanced gel elasticity. Amplitude sweep results showed that crossover points increased with Carbopol® 974P concentration indicating greater polymer network strength and structural integrity under applied stress. Texture analysis revealed that increasing Carbopol® 974P concentration led to increased hardness (G2: 1.1 ± 0.00 g, G5: 2.8 ± 0.17) and decreased adhesiveness (G2: 1.25 ± 0.21 g, G5: -16.17 ± 0.25 g). Cohesiveness values remained stable suggesting consistent internal bonding. IVPT results showed similar permeation profiles between G1 and G2, and relatively higher cumulative permeation were observed for G5 that has the highest concentration of Carbopol® 974P compared to the reference formulation G1. The results suggest that Q2 changes in Carbopol® 974P up to 25% relative to the reference (0.5% w/w) may impact the drug product’s Q3, specially rheology. The potential impact of the Q3 changes on performance are under investigation. (Figure 2).
Conclusion: The current study suggests that, for the diclofenac sodium topical gels evaluated, Q2 changes in Carbopol® 974P at or greater than ±10% relative to the reference (0.5% w/w) can impact Q3 attributes of diclofenac sodium topical gels such as viscosity, elasticity, and texture. The impact of the differences in Q3 compared to the reference (0.5% w/w) on performance is current under investigation.
References: 1. FDA, CDER, In Vitro Release Test Studies for Topical Drug Products Submitted in ANDAs Guidance for Industry DRAFT GUIDANCE. https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs (accessed March 28, 2025).
2. FDA, CDER, In Vitro Permeation Test Studies for Topical Drug Products Submitted in ANDAs Guidance for Industry DRAFT GUIDANCE. https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs (accessed March 28, 2025).
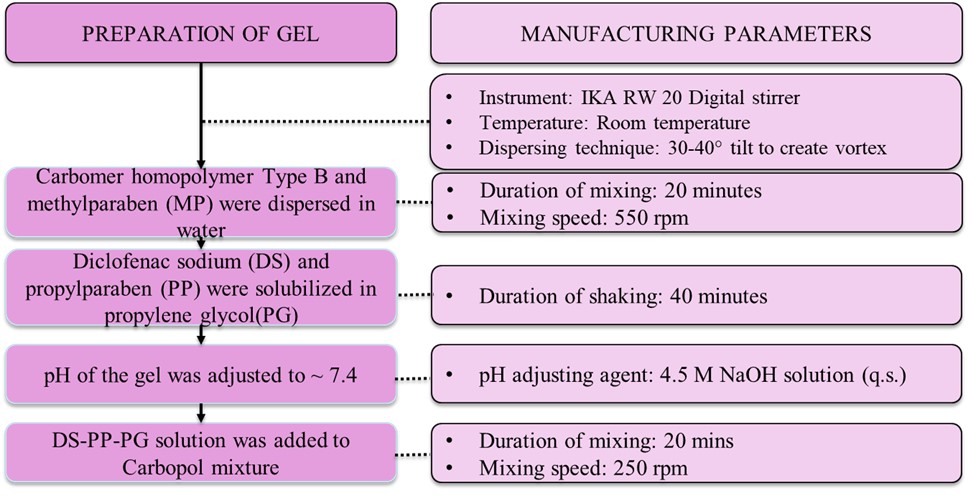 Figure 1. Manufacturing method of diclofenac sodium topical gels containing Carbopol® 974P (G1-G5)
Figure 1. Manufacturing method of diclofenac sodium topical gels containing Carbopol® 974P (G1-G5)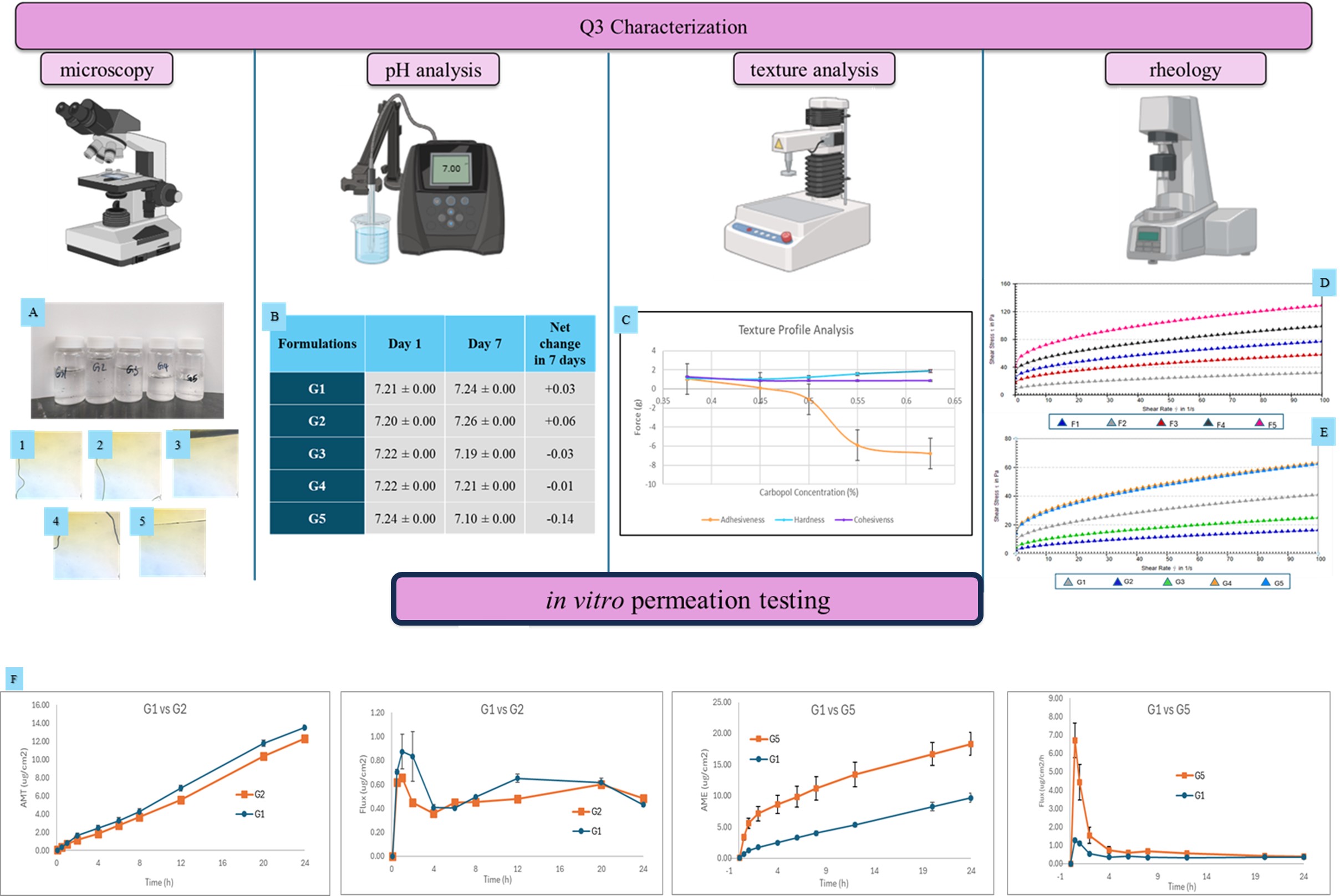 Figure 2: Q3 characterization and IVPT results for diclofenac sodium topical gels (G1-G5). (A) Visual appearance and microscopic images; (B) pH monitoring (n=3); (C) texture profile (Mean ± SD, n=3); (D) shear stress vs/ shear rate rheology profiles (data represents mean values, n=3); (E) viscosity vs/ shear rate rheology profiles (data represents mean values, n=3); (F) IVPT drug permeation profiles and flux profiles (Mean ± SE, 3 donors, 4 replicates per donor)
Figure 2: Q3 characterization and IVPT results for diclofenac sodium topical gels (G1-G5). (A) Visual appearance and microscopic images; (B) pH monitoring (n=3); (C) texture profile (Mean ± SD, n=3); (D) shear stress vs/ shear rate rheology profiles (data represents mean values, n=3); (E) viscosity vs/ shear rate rheology profiles (data represents mean values, n=3); (F) IVPT drug permeation profiles and flux profiles (Mean ± SE, 3 donors, 4 replicates per donor)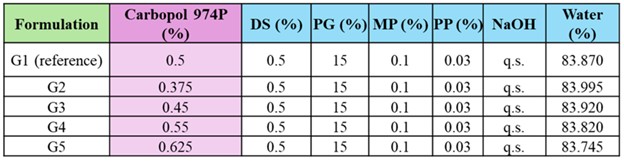 Table 1. Formulation composition of diclofenac sodium topical gels containing Carbopol® 974P (G1-G5)
Table 1. Formulation composition of diclofenac sodium topical gels containing Carbopol® 974P (G1-G5)