Manufacturing and Analytical Characterization - Chemical
(T1130-02-13) Microscope-Enabled Disc Dissolution System: Concordance between Drug and Polymer Dissolution from an Amorphous Solid Dispersion Disc and Visual Disc Degradation
Tuesday, November 11, 2025
11:30 AM - 12:30 PM CT
- SM
Shuaiqian Men, BS
Research Assistant
University of Maryland
Baltimore, Maryland, United States - SM
Shuaiqian Men, BS
Research Assistant
University of Maryland
Baltimore, Maryland, United States 
James E. Polli, Ph.D.
Professor and Ralph F. Shangraw/Noxell Endowed Chair in Industrial Pharmacy and Pharmaceutics
University of Maryland
Baltimore, Maryland, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Objectives were 1) to design and build a microscope-enabled disc dissolution system (MeDDiS) with a 900 mL dissolution volume and 2) assess the ability of MeDDiS imaging of dissolving discs to provide concordance with measured drug and polymer dissolution profiles from the dissolving discs. ASD discs containing ritonavir (5–50 %) and PVPVA were fabricated using a lab-scaled melting and fusion method, denoted vacuum compression molding (VCM). ASD discs were then subjected to in vitro dissolution using MeDDiS, where disc diameter was quantified with time. Ritonavir and PVPVA release were also measured. Results indicate concordance between imaging and measured dissolution. Overall, studies here describe a MeDDiS which has promised to anticipate drug and polymer dissolution, via imaging of dissolving discs, above and below the ASD drug load cliff.
Methods: Ritonavir and copovidone (PVPVA) were sourced for experiments, with 3D-printed holders and accessories manufactured for a custom microscope-enabled disc dissolution system (MeDDiS). A large 1L crystallization vessel was heated, stirred, and monitored for temperature, while ASD discs (RTV/PVPVA blends) were made by cryogenic milling and vacuum compression molding. Disc dissolution was imaged microscopically and digitally quantified over time. Drug and polymer release were assessed by HPLC, while imaging measured disc volume loss. Polarized light microscopy checked for crystallinity in the colloid, confirming ASD amorphousness during dissolution.
Results: The MeDDiS system was effectively designed to enable microscopic imaging during ASD disc dissolution with a large 900 mL media volume. The configuration, featuring a wide, shallow vessel and custom 3D-printed holder, allowed optimal sample stabilization and imaging through the solution. Medium flow dye tests demonstrated rapid, homogeneous mixing within 5 seconds at 500 rpm, suggesting efficient medium distribution for dissolution studies. Dissolution profiles, measured by HPLC and SEC-UV, showed that at low drug loads (5–25 % RTV), both ritonavir (RTV) and polymer (PVPVA) were released congruently and nearly completely, plateauing at roughly 90 % by 120 minutes. Images visually confirmed the discs’ nearly complete disappearance, with only traces of colloid at the end. However, a dramatic “cliff effect” was observed between 25 and 30 % drug load: at 30 % drug loading, polymer dissolved significantly faster than drug (about 80 % vs. 30 % over 240–300 min), with most RTV remaining as undissolved, drug-rich colloid—a sign of liquid–liquid phase separation (LLPS). At even higher drug loads (35 % and above), both drug and polymer released were very low and incongruent; most of the disc remained undissolved as a glassy core, and release plateaued at 2 % RTV and 6 % PVPVA. Images showed little change after the initial 10 minutes for high-drug discs. For discs well below the “cliff,” image analysis and direct measurement agreed: disc volume shrank linearly, and dissolution finished within 120 minutes, matching observed drug and polymer release. For discs near or above the “cliff,” image-based predictions tended to overestimate actual release, because this method could not distinguish undissolved colloid from true solution. Still, both approaches captured the abrupt transition, accurately predicting the composition where the cliff effect emerged. Polarized light microscopy on the colloid formed at both low and high drug loads showed no evidence of crystallinity, confirming the undissolved colloid was amorphous and drug-rich, consistent with LLPS rather than crystallization. Together, MeDDiS enabled comprehensive, parallel chemical and visual tracking of ASD disc dissolution, clearly revealing the sharp change in dissolution behavior above a critical drug load.
Conclusion: The current study aimed at designing MeDDiS, a novel screening method for drug and polymer pairs, with a focus on dissolution and imaging, including the ability of MeDDiS to predict RTV and PVPVA release profiles from disc imaging only. MeDDiS overcomes the limitation of narrow microscope working space and provides VCM disc dissolution into a 900 mL, as well as simultaneous disc imaging. The dissolution and visual observation results show that image analysis results were in agreement with HPLC quantification of drug and polymer release. MeDDiS imaging has promised to estimate drug and polymer release profile, both above and below the drug load cliff.
References: 1. Chiou WL, Riegelman S. Pharmaceutical applications of solid dispersion systems. J Pharm Sci. 1971;60:1281–302.
2. Purohit HS, Taylor LS. Phase Behavior of Ritonavir Amorphous Solid Dispersions during Hydration and Dissolution. Pharm Res. 2017;34:2842–61.
3. Indulkar AS, Lou X, Zhang GGZ, Taylor LS. Insights into the Dissolution Mechanism of Ritonavir–Copovidone Amorphous Solid Dispersions: Importance of Congruent Release for Enhanced Performance. Mol Pharmaceutics. 2019;16:1327–39.
4. Bochmann ES, Steidel A, Rosenblatt KM, Gessner D, Liepold B. Assessment of the amorphous solid dispersion erosion behavior following a novel small-scale predictive approach. European Journal of Pharmaceutical Sciences. 2021;158:105682.
5. Dohrn S, Kyeremateng SO, Bochmann E, Sobich E, Wahl A, Liepold B, et al. Thermodynamic Modeling of the Amorphous Solid Dispersion-Water Interfacial Layer and Its Impact on the Release Mechanism. Pharmaceutics. 2023;15.
6. Taylor LS. - Dissolution Mechanisms of Amorphous Solid Dispersions: A Close Look at the Dissolution Interface. 2023;20. Available from: - https://doi.org/10.1021/acs.molpharmaceut.3c00020
7. Miller-Chou BA, Koenig JL. A review of polymer dissolution. Progress in Polymer Science. 2003;28:1223–70.
8. Krummnow A, Danzer A, Voges K, Dohrn S, Kyeremateng SO, Degenhardt M, et al. Explaining the Release Mechanism of Ritonavir/PVPVA Amorphous Solid Dispersions. Pharmaceutics. 2022;14.
9. Treffer D, Troiss A, Khinast J. A novel tool to standardize rheology testing of molten polymers for pharmaceutical applications. Int J Pharm. 2015;495:474–81.
Acknowledgements: Declaration of competing interest: There are no conflicts of interest for these authors to declare. This work was funded by the generosity of Marilyn Shangraw. J.E.P. is the Ralph F. Shangraw Endowed Professor in Industrial Pharmacy and Pharmaceutics.
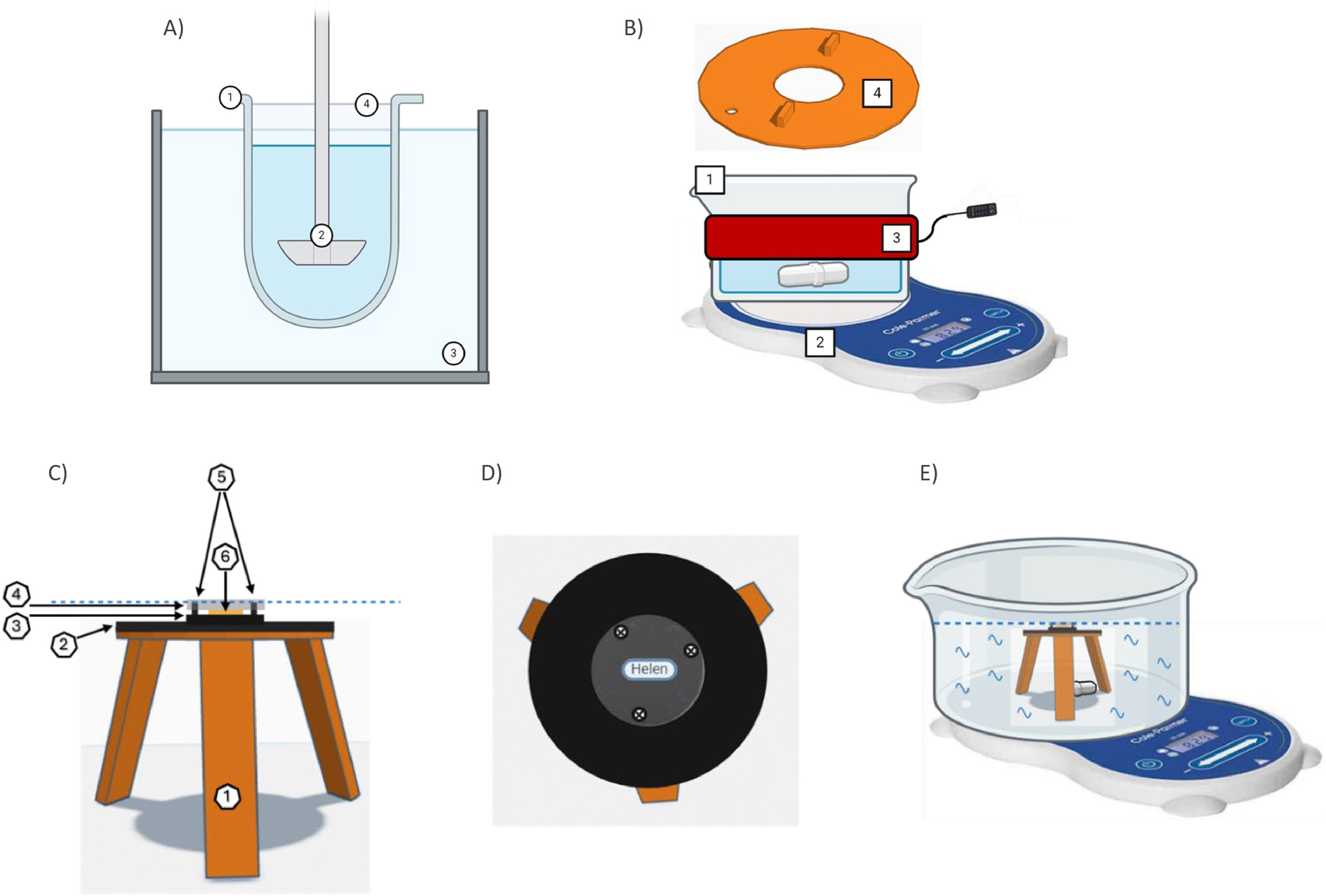 Fig. 1. Comparison of MeDDiS to USP II apparatus, and diagram of MeDDiS disc holder and ASD sandwich components. Panel A shows USP II apparatus. Panel B illustrates some MeDDiS components. Differences include: 1. MeDDiS employs a wide 1 L crystallization dish, allowing fit underneath microscope lens (not shown), in place of the compendial dissolution vessel. 2. MeDDiS uses a stir bar and stirring plate in place of a paddle. 3. MeDDiS employs a beaker heating wrap in place of a water heating bath. 4. MeDDiS uses a 3D printed lid (with hole for temperature monitoring) that allows microscope imaging (from above) of a centrally positioned ASD disc (not shown). Panel C is a side view of ASD disc holder, which consists of 1) 3D printed, 3-legged holder which sits in the dissolution vessel, 2) cuttable magnetic sheets, 3) 1-inch black acrylic plastic saucer below ASD disc (with name sticker to assess visual transparency of ASD disc), 4) 1-inch clear acrylic plastic saucer to protect ASD disc top from dissolution media, 5) mini-screws to compress to clear plastic saucer towards black acrylic plastic saucer and hence protect ASD disc top and bottom from dissolution media, and 6) ASD disc stabilized with silicone grease. The black acrylic saucer, the ASD disc, and the clear acrylic saucer are held together via the mini-screws and form the ASD sandwich. A magnetic sheet was fixed to the bottom of the black acrylic plastic saucer (i.e. the ASD sandwich) to stabilize it onto the center of the holder, via magnetic force. Panel D is a top view of ASD disc holder and ASD sandwich without an ASD disc. Name sticker “Helen” is visibly obscured when ASD disc is in a gel state. Panel E highlights the positioning of holder in the center of the dissolution vessel (secured with jelly tape) with stir bar on stirring plate. The dotted blue dotted line in panels A and C indicates the dissolution media level, which did not exceed the top clear acrylic plastic saucer, allowing the above microscope (not drawn) to focus on the dissolving ASD discs throughout the study.
Fig. 1. Comparison of MeDDiS to USP II apparatus, and diagram of MeDDiS disc holder and ASD sandwich components. Panel A shows USP II apparatus. Panel B illustrates some MeDDiS components. Differences include: 1. MeDDiS employs a wide 1 L crystallization dish, allowing fit underneath microscope lens (not shown), in place of the compendial dissolution vessel. 2. MeDDiS uses a stir bar and stirring plate in place of a paddle. 3. MeDDiS employs a beaker heating wrap in place of a water heating bath. 4. MeDDiS uses a 3D printed lid (with hole for temperature monitoring) that allows microscope imaging (from above) of a centrally positioned ASD disc (not shown). Panel C is a side view of ASD disc holder, which consists of 1) 3D printed, 3-legged holder which sits in the dissolution vessel, 2) cuttable magnetic sheets, 3) 1-inch black acrylic plastic saucer below ASD disc (with name sticker to assess visual transparency of ASD disc), 4) 1-inch clear acrylic plastic saucer to protect ASD disc top from dissolution media, 5) mini-screws to compress to clear plastic saucer towards black acrylic plastic saucer and hence protect ASD disc top and bottom from dissolution media, and 6) ASD disc stabilized with silicone grease. The black acrylic saucer, the ASD disc, and the clear acrylic saucer are held together via the mini-screws and form the ASD sandwich. A magnetic sheet was fixed to the bottom of the black acrylic plastic saucer (i.e. the ASD sandwich) to stabilize it onto the center of the holder, via magnetic force. Panel D is a top view of ASD disc holder and ASD sandwich without an ASD disc. Name sticker “Helen” is visibly obscured when ASD disc is in a gel state. Panel E highlights the positioning of holder in the center of the dissolution vessel (secured with jelly tape) with stir bar on stirring plate. The dotted blue dotted line in panels A and C indicates the dissolution media level, which did not exceed the top clear acrylic plastic saucer, allowing the above microscope (not drawn) to focus on the dissolving ASD discs throughout the study.
.jpg) Fig. 2. Digital microscope images of four discs undergoing dissolution in MeDDiS. Four discs had compositions 20DL/80PL, 25DL/75PL, 30DL/70PL, and 40DL/60PL. A) 20DL/80PL was chosen as an example of low DL. B) 25DL/75DL showed slightly slower disc loss than discs with low DL. C) 30DL/70PL exhibited a large amount of remaining white gel-like undissolved particles. D) 40DL/60PL exemplified high DL where >80 % dry glassy core was undissolved at the end of the study at 300 min.
Fig. 2. Digital microscope images of four discs undergoing dissolution in MeDDiS. Four discs had compositions 20DL/80PL, 25DL/75PL, 30DL/70PL, and 40DL/60PL. A) 20DL/80PL was chosen as an example of low DL. B) 25DL/75DL showed slightly slower disc loss than discs with low DL. C) 30DL/70PL exhibited a large amount of remaining white gel-like undissolved particles. D) 40DL/60PL exemplified high DL where >80 % dry glassy core was undissolved at the end of the study at 300 min.
.jpg) Fig. 3. Comparison of observed and predicted RTV and PVPVA profiles from discs with different DLs/PLs. A) 5DL/95PL. B) 10DL/90PL. C) 15DL/85PL. D) 20DL/80DL. E) 25DL/75DL. F) 30DL/70PL. G) 35DL/65PL. H) 40DL/60PL. I) 45DL/55PL. J) 50DL/50PL. Observed profiles were measured using HPLC and SEC-UV for drug and polymer respectively. Predicted profiles were from microscope imaging. Error bar is SD from n = 3.
Fig. 3. Comparison of observed and predicted RTV and PVPVA profiles from discs with different DLs/PLs. A) 5DL/95PL. B) 10DL/90PL. C) 15DL/85PL. D) 20DL/80DL. E) 25DL/75DL. F) 30DL/70PL. G) 35DL/65PL. H) 40DL/60PL. I) 45DL/55PL. J) 50DL/50PL. Observed profiles were measured using HPLC and SEC-UV for drug and polymer respectively. Predicted profiles were from microscope imaging. Error bar is SD from n = 3.
Methods: Ritonavir and copovidone (PVPVA) were sourced for experiments, with 3D-printed holders and accessories manufactured for a custom microscope-enabled disc dissolution system (MeDDiS). A large 1L crystallization vessel was heated, stirred, and monitored for temperature, while ASD discs (RTV/PVPVA blends) were made by cryogenic milling and vacuum compression molding. Disc dissolution was imaged microscopically and digitally quantified over time. Drug and polymer release were assessed by HPLC, while imaging measured disc volume loss. Polarized light microscopy checked for crystallinity in the colloid, confirming ASD amorphousness during dissolution.
Results: The MeDDiS system was effectively designed to enable microscopic imaging during ASD disc dissolution with a large 900 mL media volume. The configuration, featuring a wide, shallow vessel and custom 3D-printed holder, allowed optimal sample stabilization and imaging through the solution. Medium flow dye tests demonstrated rapid, homogeneous mixing within 5 seconds at 500 rpm, suggesting efficient medium distribution for dissolution studies. Dissolution profiles, measured by HPLC and SEC-UV, showed that at low drug loads (5–25 % RTV), both ritonavir (RTV) and polymer (PVPVA) were released congruently and nearly completely, plateauing at roughly 90 % by 120 minutes. Images visually confirmed the discs’ nearly complete disappearance, with only traces of colloid at the end. However, a dramatic “cliff effect” was observed between 25 and 30 % drug load: at 30 % drug loading, polymer dissolved significantly faster than drug (about 80 % vs. 30 % over 240–300 min), with most RTV remaining as undissolved, drug-rich colloid—a sign of liquid–liquid phase separation (LLPS). At even higher drug loads (35 % and above), both drug and polymer released were very low and incongruent; most of the disc remained undissolved as a glassy core, and release plateaued at 2 % RTV and 6 % PVPVA. Images showed little change after the initial 10 minutes for high-drug discs. For discs well below the “cliff,” image analysis and direct measurement agreed: disc volume shrank linearly, and dissolution finished within 120 minutes, matching observed drug and polymer release. For discs near or above the “cliff,” image-based predictions tended to overestimate actual release, because this method could not distinguish undissolved colloid from true solution. Still, both approaches captured the abrupt transition, accurately predicting the composition where the cliff effect emerged. Polarized light microscopy on the colloid formed at both low and high drug loads showed no evidence of crystallinity, confirming the undissolved colloid was amorphous and drug-rich, consistent with LLPS rather than crystallization. Together, MeDDiS enabled comprehensive, parallel chemical and visual tracking of ASD disc dissolution, clearly revealing the sharp change in dissolution behavior above a critical drug load.
Conclusion: The current study aimed at designing MeDDiS, a novel screening method for drug and polymer pairs, with a focus on dissolution and imaging, including the ability of MeDDiS to predict RTV and PVPVA release profiles from disc imaging only. MeDDiS overcomes the limitation of narrow microscope working space and provides VCM disc dissolution into a 900 mL, as well as simultaneous disc imaging. The dissolution and visual observation results show that image analysis results were in agreement with HPLC quantification of drug and polymer release. MeDDiS imaging has promised to estimate drug and polymer release profile, both above and below the drug load cliff.
References: 1. Chiou WL, Riegelman S. Pharmaceutical applications of solid dispersion systems. J Pharm Sci. 1971;60:1281–302.
2. Purohit HS, Taylor LS. Phase Behavior of Ritonavir Amorphous Solid Dispersions during Hydration and Dissolution. Pharm Res. 2017;34:2842–61.
3. Indulkar AS, Lou X, Zhang GGZ, Taylor LS. Insights into the Dissolution Mechanism of Ritonavir–Copovidone Amorphous Solid Dispersions: Importance of Congruent Release for Enhanced Performance. Mol Pharmaceutics. 2019;16:1327–39.
4. Bochmann ES, Steidel A, Rosenblatt KM, Gessner D, Liepold B. Assessment of the amorphous solid dispersion erosion behavior following a novel small-scale predictive approach. European Journal of Pharmaceutical Sciences. 2021;158:105682.
5. Dohrn S, Kyeremateng SO, Bochmann E, Sobich E, Wahl A, Liepold B, et al. Thermodynamic Modeling of the Amorphous Solid Dispersion-Water Interfacial Layer and Its Impact on the Release Mechanism. Pharmaceutics. 2023;15.
6. Taylor LS. - Dissolution Mechanisms of Amorphous Solid Dispersions: A Close Look at the Dissolution Interface. 2023;20. Available from: - https://doi.org/10.1021/acs.molpharmaceut.3c00020
7. Miller-Chou BA, Koenig JL. A review of polymer dissolution. Progress in Polymer Science. 2003;28:1223–70.
8. Krummnow A, Danzer A, Voges K, Dohrn S, Kyeremateng SO, Degenhardt M, et al. Explaining the Release Mechanism of Ritonavir/PVPVA Amorphous Solid Dispersions. Pharmaceutics. 2022;14.
9. Treffer D, Troiss A, Khinast J. A novel tool to standardize rheology testing of molten polymers for pharmaceutical applications. Int J Pharm. 2015;495:474–81.
Acknowledgements: Declaration of competing interest: There are no conflicts of interest for these authors to declare. This work was funded by the generosity of Marilyn Shangraw. J.E.P. is the Ralph F. Shangraw Endowed Professor in Industrial Pharmacy and Pharmaceutics.
 Fig. 1. Comparison of MeDDiS to USP II apparatus, and diagram of MeDDiS disc holder and ASD sandwich components. Panel A shows USP II apparatus. Panel B illustrates some MeDDiS components. Differences include: 1. MeDDiS employs a wide 1 L crystallization dish, allowing fit underneath microscope lens (not shown), in place of the compendial dissolution vessel. 2. MeDDiS uses a stir bar and stirring plate in place of a paddle. 3. MeDDiS employs a beaker heating wrap in place of a water heating bath. 4. MeDDiS uses a 3D printed lid (with hole for temperature monitoring) that allows microscope imaging (from above) of a centrally positioned ASD disc (not shown). Panel C is a side view of ASD disc holder, which consists of 1) 3D printed, 3-legged holder which sits in the dissolution vessel, 2) cuttable magnetic sheets, 3) 1-inch black acrylic plastic saucer below ASD disc (with name sticker to assess visual transparency of ASD disc), 4) 1-inch clear acrylic plastic saucer to protect ASD disc top from dissolution media, 5) mini-screws to compress to clear plastic saucer towards black acrylic plastic saucer and hence protect ASD disc top and bottom from dissolution media, and 6) ASD disc stabilized with silicone grease. The black acrylic saucer, the ASD disc, and the clear acrylic saucer are held together via the mini-screws and form the ASD sandwich. A magnetic sheet was fixed to the bottom of the black acrylic plastic saucer (i.e. the ASD sandwich) to stabilize it onto the center of the holder, via magnetic force. Panel D is a top view of ASD disc holder and ASD sandwich without an ASD disc. Name sticker “Helen” is visibly obscured when ASD disc is in a gel state. Panel E highlights the positioning of holder in the center of the dissolution vessel (secured with jelly tape) with stir bar on stirring plate. The dotted blue dotted line in panels A and C indicates the dissolution media level, which did not exceed the top clear acrylic plastic saucer, allowing the above microscope (not drawn) to focus on the dissolving ASD discs throughout the study.
Fig. 1. Comparison of MeDDiS to USP II apparatus, and diagram of MeDDiS disc holder and ASD sandwich components. Panel A shows USP II apparatus. Panel B illustrates some MeDDiS components. Differences include: 1. MeDDiS employs a wide 1 L crystallization dish, allowing fit underneath microscope lens (not shown), in place of the compendial dissolution vessel. 2. MeDDiS uses a stir bar and stirring plate in place of a paddle. 3. MeDDiS employs a beaker heating wrap in place of a water heating bath. 4. MeDDiS uses a 3D printed lid (with hole for temperature monitoring) that allows microscope imaging (from above) of a centrally positioned ASD disc (not shown). Panel C is a side view of ASD disc holder, which consists of 1) 3D printed, 3-legged holder which sits in the dissolution vessel, 2) cuttable magnetic sheets, 3) 1-inch black acrylic plastic saucer below ASD disc (with name sticker to assess visual transparency of ASD disc), 4) 1-inch clear acrylic plastic saucer to protect ASD disc top from dissolution media, 5) mini-screws to compress to clear plastic saucer towards black acrylic plastic saucer and hence protect ASD disc top and bottom from dissolution media, and 6) ASD disc stabilized with silicone grease. The black acrylic saucer, the ASD disc, and the clear acrylic saucer are held together via the mini-screws and form the ASD sandwich. A magnetic sheet was fixed to the bottom of the black acrylic plastic saucer (i.e. the ASD sandwich) to stabilize it onto the center of the holder, via magnetic force. Panel D is a top view of ASD disc holder and ASD sandwich without an ASD disc. Name sticker “Helen” is visibly obscured when ASD disc is in a gel state. Panel E highlights the positioning of holder in the center of the dissolution vessel (secured with jelly tape) with stir bar on stirring plate. The dotted blue dotted line in panels A and C indicates the dissolution media level, which did not exceed the top clear acrylic plastic saucer, allowing the above microscope (not drawn) to focus on the dissolving ASD discs throughout the study..jpg) Fig. 2. Digital microscope images of four discs undergoing dissolution in MeDDiS. Four discs had compositions 20DL/80PL, 25DL/75PL, 30DL/70PL, and 40DL/60PL. A) 20DL/80PL was chosen as an example of low DL. B) 25DL/75DL showed slightly slower disc loss than discs with low DL. C) 30DL/70PL exhibited a large amount of remaining white gel-like undissolved particles. D) 40DL/60PL exemplified high DL where >80 % dry glassy core was undissolved at the end of the study at 300 min.
Fig. 2. Digital microscope images of four discs undergoing dissolution in MeDDiS. Four discs had compositions 20DL/80PL, 25DL/75PL, 30DL/70PL, and 40DL/60PL. A) 20DL/80PL was chosen as an example of low DL. B) 25DL/75DL showed slightly slower disc loss than discs with low DL. C) 30DL/70PL exhibited a large amount of remaining white gel-like undissolved particles. D) 40DL/60PL exemplified high DL where >80 % dry glassy core was undissolved at the end of the study at 300 min..jpg) Fig. 3. Comparison of observed and predicted RTV and PVPVA profiles from discs with different DLs/PLs. A) 5DL/95PL. B) 10DL/90PL. C) 15DL/85PL. D) 20DL/80DL. E) 25DL/75DL. F) 30DL/70PL. G) 35DL/65PL. H) 40DL/60PL. I) 45DL/55PL. J) 50DL/50PL. Observed profiles were measured using HPLC and SEC-UV for drug and polymer respectively. Predicted profiles were from microscope imaging. Error bar is SD from n = 3.
Fig. 3. Comparison of observed and predicted RTV and PVPVA profiles from discs with different DLs/PLs. A) 5DL/95PL. B) 10DL/90PL. C) 15DL/85PL. D) 20DL/80DL. E) 25DL/75DL. F) 30DL/70PL. G) 35DL/65PL. H) 40DL/60PL. I) 45DL/55PL. J) 50DL/50PL. Observed profiles were measured using HPLC and SEC-UV for drug and polymer respectively. Predicted profiles were from microscope imaging. Error bar is SD from n = 3.