Preclinical, Clinical, and Translational Sciences
(T1230-05-30) Sensory Panel Tests for Topical Semi-Solid Creams: Correlation between Sensorial Perceptions and Product Critical Quality Attributes

Yousuf Hussain Mohammed, Ph.D.
Associate Professor
University of Queensland
Brisbane, Queensland, Australia
Yousuf Hussain Mohammed, Ph.D.
Associate Professor
University of Queensland
Brisbane, Queensland, Australia- KP
Khanh Phan, Ph.D. (she/her/hers)
Postdoc
University of Queensland
BRISBANE, Queensland, Australia - VL
Vânia R. Leite-Silva, Ph.D.
Professor
Federal University of Sao Paulo
Sao Paulo, Sao Paulo, Brazil - SP
Sangeeta Prakash, Ph.D. (she/her/hers)
Associate Professor
University of Queensland
Brisbane, Queensland, Australia - DL
David Liu, PhD
Postdoc
University of Queensland
Brisbane, Queensland, Australia 
Tannaz Ramezanli, Ph.D.
Pharmacologist
US Food and Drug Administration
Maryland, District of Columbia, United States
Priyanka Ghosh, Ph.D.
Lead Pharmacologist at Office of Research and Standards
US Food and Drug Administration
Maryland, Maryland, United States
Sam G. Raney, Ph.D. (he/him/his)
Associate Director
US Food and Drug Administration
Maryland, District of Columbia, United States
Markham C. C. Luke, Ph.D.
Director
US Food and Drug Administration
Maryland, District of Columbia, United States- MR
Michael Roberts, Ph.D.
Professor
University of Queensland
Brisbane, Queensland, Australia
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: To perform this work, 8 creams with two blinded replicates, with different concentrations of ammonium lauryl sulphate (ALS), mineral oil, cetostearyl alcohol and poloxamer were selected from 25 lab manufactured cream formulations, by using statistical analyses such as principal component analysis (PCA) and Hierarchical clustering method to compare and group CQAs data (Figure 1). For in vitro characterization of CQAs, measurements of rheological parameters of creams (n=3) were carried out using an Anton-Paar rheometer system with the shear, temperature and strain sweep tests. Textural properties of cream samples (n=5) were examined by applying the texture profile analysis with a CTX Brookfield texture analyzer. Frictional property of creams (n=3) was determined by using the Anton-Paar rheometer system equipped with a tribology cell and a customized sample holder. For in vivo sensory panel test, skin biophysical properties of 32 subjects, 23 females and 9 males, aged between 18 and 70 years, (Ethics ID number: 2020/HE001995) were first tested using a non-invasive Courage + Khazaka (C+K) GmbH electronic equipment. Then, the subjects were trained on concepts and assessment criteria of the 11 different sensory attributes (i.e., spreadability, cooling sensation, immediate white residue, immediate shine, immediate oiliness, stickiness, smoothness, white residue, residue shine, residue oiliness, and dry touch). These visual and touch attributes can be classified as during and after-feel sensations. To start the panel test, at time 0, onto a marked area of the forearm (19.6 cm2) of each subject, 25 µL of each cream sample was placed and spread by subject’s forefinger at rotational speed of about 1 circle/s for 15 seconds. After 15 seconds, subjects stopped spreading, and assessed cooling sensation, immediate white residue, immediate shine and immediate oiliness. The after-feel attributes of stickiness and smoothness were evaluated after waiting for 1 and 1.5 minutes, respectively. Finally, after 2 minutes, subjects assessed white residue, residual shine, residual oiliness, and dry touch. The intensity of each cream sensorial attribute was rated using a continuous 1-9 scale, representing from very low (1) to very high (9) intensity.
Results: Skin biophysical parameters (by C+K instrument) of the 32 female and male subjects (with a wide age range) exhibited high interindividual variability, which represents a true sample from the general population. The recorded intensity scores of 11 sensory attributes for the 8 examined topical creams are depicted as spider graphs. Although subjects had differences in skin biophysical properties, they perceived sensorial attributes of the two sets of blinded replicates (named as SC2.Ref-1, SC2.Ref-2 and SC2.18-1, SC2.18-2), consistently. To clearly observe the impact of inactive ingredient concentration (Q2) changes on CQAs and sensorial properties of the samples, the outcomes were divided into 4 groups for ALS (Figure 2a), cetostearyl alcohol (Figure 2b), mineral oil (Figure 2c) and poloxamer variants (Figure 2d). The participants perceived notable differences in several sensory attributes including spreadability, immediate white residue, immediate shine, immediate oiliness and stickiness when comparing the reference formulation to ALS and mineral oil variants. These perceived differences may be linked to variations in formulation composition and corresponding in vitro characterization results. As shown in Figure 1b, these variants, are positioned at considerable distances from the reference formulation and occupy different quadrants of the PCA plot. The greater separation suggests more significant differences in CQAs, such as rheological, tribological behaviour and textural properties, between the reference and composition variant formulations, which are more likely to be perceived differently by human subjects.
Conclusion: The obtained data demonstrate that substantial differences in some of CQAs are likely to be perceptible by human subjects. The findings also suggest that it may be possible to understand and predict some potential differences in sensorial attributes between two topical formulations from their physicochemical and structural characteristics assessed instrumently.
Acknowledgements: This project is supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of a financial assistance award [U01FD006700] totaling $1,250,000 with 100 percent funded by FDA/HHS. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by FDA, or the U.S. Government.
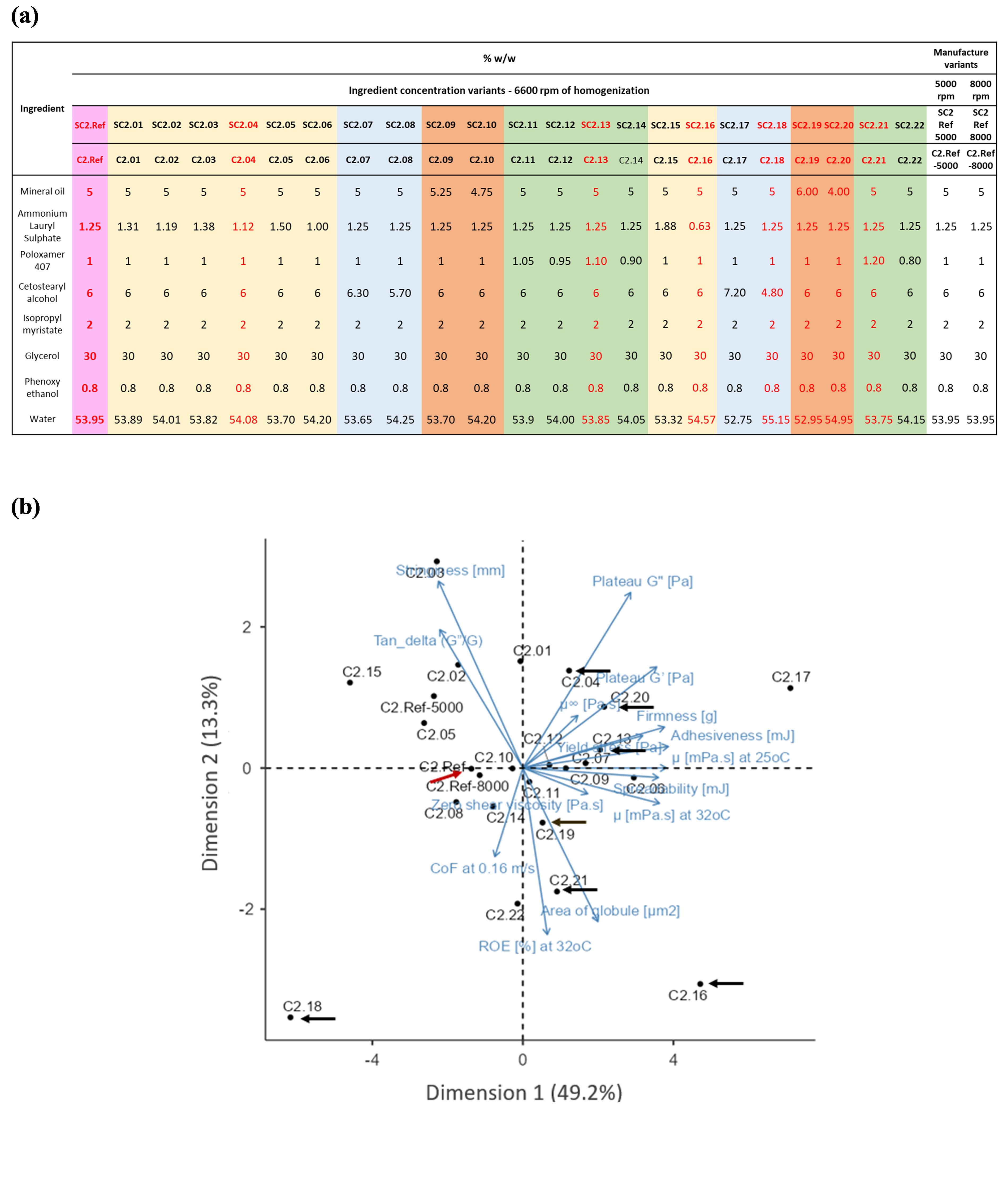 Figure 1. (a) Formulation table of 25 topical creams varied in %w/w of ammonium lauryl sulphate (highlighted in yellow), cetostearyl alcohol (highlighted in blue), poloxamer (highlighted in green), and mineral oil (highlighted in orange) composition; (b) Eight cream formulations including SC2.Ref (the reference formulation, highlighted in pink), SC2.04, SC2.13, SC2.16, SC2.18, SC2.19, SC2.20 and SC2.21 (in red font in 1.(a)), identified as C2.Ref, C2.04, C2.13, C2.16, C2.18, C2.19, C2.20 and C2.21, respectively, were selected out of 25 formulations for the sensory panel study by using statistical analyses, according to their formulation composition and a subset of CQAs.
Figure 1. (a) Formulation table of 25 topical creams varied in %w/w of ammonium lauryl sulphate (highlighted in yellow), cetostearyl alcohol (highlighted in blue), poloxamer (highlighted in green), and mineral oil (highlighted in orange) composition; (b) Eight cream formulations including SC2.Ref (the reference formulation, highlighted in pink), SC2.04, SC2.13, SC2.16, SC2.18, SC2.19, SC2.20 and SC2.21 (in red font in 1.(a)), identified as C2.Ref, C2.04, C2.13, C2.16, C2.18, C2.19, C2.20 and C2.21, respectively, were selected out of 25 formulations for the sensory panel study by using statistical analyses, according to their formulation composition and a subset of CQAs.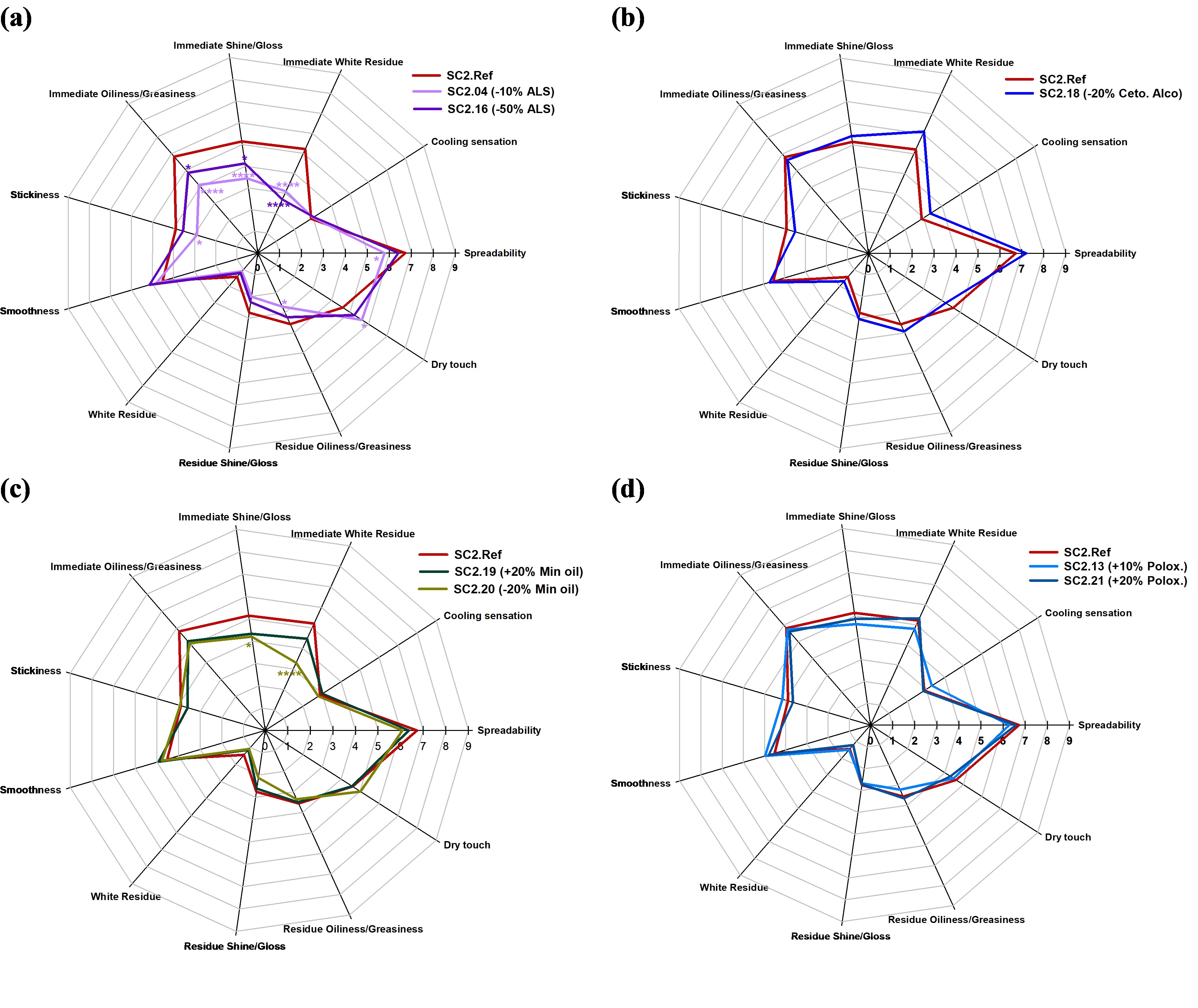 Figure 2. Spider diagram of sensory perceptions of spreadability, cooling sensation, immediate white residue, immediate shine, immediate oiliness, stickiness, smoothness, white residue, residue shine, residue oiliness, and dry touch assessed using 9-point hedonic scale of the 4 groups of formulation variants: (a) ammonium lauryl sulphate (ALS), (b) cetostearyl alcohol (Ceto. Alco.), (c) mineral oil (Min oil), (d) poloxamer (Polox.) (formulations presented in Figure 1a), showing correlations among sensory perceptions of the cream formulation composition differences in -/+ % w/w compared with that of the reference (Ref) formulation. The number of “*” summarizes the p values/significant levels: without “*” meaning p>0.05 or no significant difference, with “*” significant difference at p<0.05, “**” significant difference at p<0.01, “***” significant difference at p<0.0005, and “****” significant difference at p<0.0001 between the cream formulations.
Figure 2. Spider diagram of sensory perceptions of spreadability, cooling sensation, immediate white residue, immediate shine, immediate oiliness, stickiness, smoothness, white residue, residue shine, residue oiliness, and dry touch assessed using 9-point hedonic scale of the 4 groups of formulation variants: (a) ammonium lauryl sulphate (ALS), (b) cetostearyl alcohol (Ceto. Alco.), (c) mineral oil (Min oil), (d) poloxamer (Polox.) (formulations presented in Figure 1a), showing correlations among sensory perceptions of the cream formulation composition differences in -/+ % w/w compared with that of the reference (Ref) formulation. The number of “*” summarizes the p values/significant levels: without “*” meaning p>0.05 or no significant difference, with “*” significant difference at p<0.05, “**” significant difference at p<0.01, “***” significant difference at p<0.0005, and “****” significant difference at p<0.0001 between the cream formulations.