Manufacturing and Analytical Characterization - Chemical
(T1330-02-13) Role of Surface Morphological Characteristics of Substrate in Adhesion Performance of Topical and Transdermal Delivery Systems (TDS)

Yousuf Hussain Mohammed, Ph.D.
Associate Professor
University of Queensland
Brisbane, Queensland, Australia
Yousuf Hussain Mohammed, Ph.D.
Associate Professor
University of Queensland
Brisbane, Queensland, Australia- KP
Khanh Phan, Ph.D. (she/her/hers)
Postdoc
University of Queensland
BRISBANE, Queensland, Australia - JZ
Jiexin Zhu, MS
PhD Candidate
University of Queensland
BRISBANE, Queensland, Australia - MA
Masood Ali, Ph.D.
Postdoc
University of Queensland
BRISBANE, Queensland, Australia 
Priyanka Ghosh, Ph.D.
Lead Pharmacologist at Office of Research and Standards
US Food and Drug Administration
Maryland, Maryland, United States- YJ
Ying Jiang, PhD
Staff Fellow (Chemist)
US Food and Drug Administration
Maryland, District of Columbia, United States - JR
Jackson Russo, Ph.D.
Pharmacologist
US Food and Drug Administration
Maryland, District of Columbia, United States 
Markham C. C. Luke, Ph.D.
Director
US Food and Drug Administration
Maryland, District of Columbia, United States
Sam G. Raney, Ph.D. (he/him/his)
Associate Director
US Food and Drug Administration
Maryland, District of Columbia, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: Polymeric substrates were cast using 3D printed molds that were designed using Auto-CAD to have varied topography levels (M-T0 to M-T4, low to high) (Figure 1). Commercial materials (in this case referred to as DS 40:60) were used to manufacture the substrates. Micro-topographical features of the DS 40:60 substrates (n=3) were observed using a LEXT microscopic system (Olympus LEXT OLS4100, EVIDENT, Japan). Adhesion performance of an over the counter (OTC) TDS product (NicoDerm, 45mm × 45mm) (n=2) was assessed using DS 40:60 substrates and the stainless-steel substrate. The adhesion performance was also assessed with a novel peel adhesion test we developed using a texture analyzer (Brookfield CTX, AMETEK Brookfield, USA) with a standardized TDS attachment force and a 5mm/s peel rate at 180°. The force required to peel the TDS from each substrate was measured, and the area under the force-time curve (workload) representing the peel resistance was also calculated.
Results: Measured topographical parameters of developed substrates are shown in Figure 2. Both of the topographic indices, Sa (arithmetic mean of height) and Sz (sum of maximum peak height and maximum valley depth), increased when compared to M-T0, indicating that the incorporation of peaks and troughs led to a rougher and less uniform surface on the substrates. Peel adhesion results of the OTC TDS product attached on the DS 40:60 substrates and stainless-steel substrate are presented in Figure 3. As the topography level of the DS 40:60 substrates increased from M-T0 to M-T1, the peel adhesion (i.e., peak peel force and workload) increased. However, as the diameter of peak and trough circles increased on the substrates (M-T2, M-T3 and M-T4), their peel adhesion had an overall drop compared to M-T1. The increase in peak and trough diameter could make the gap between them decrease, and this forms a bigger flat surface impacting TDS adhesion. The same TDS product when attached to stainless steel substrate demonstrated better adhesion compared to the DS substrates. Both stainless steel and the M-T0 substrate are assumed to have a smooth surface; however, apart from topography, surface chemistry and physical material properties of the substrates can also play an important role in the differences in TDS adhesion tests results on these substrates.
Conclusion: Substrate topography impacts TDS adhesion and may be an important factor to incorporate into the development of skin-mimetic substrates for in vitro TDS adhesion testing. Characterization of human skin properties is underway to guide the further development of bio-relevant in vitro TDS adhesion test methods. Such test methods have the potential to help mitigate the risk of adhesion failures during product development and throughout the lifecycle of TDS products.
Acknowledgements: This project is supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of a grant (U18FD007054 -Subaward 21755 to the University of Queensland), totalling $500,000. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by FDA, or the U.S. Government. The authors thank James E. Polli, Anna S. Schwendeman, Vishalakshi Krishnan, Dana Hammell and Jennifer Dick from the Center for Research on Complex Generics (CRCG) and the CRCG Expert Committee on Adhesion Testing for TDS for their input and support in various aspects of the project.
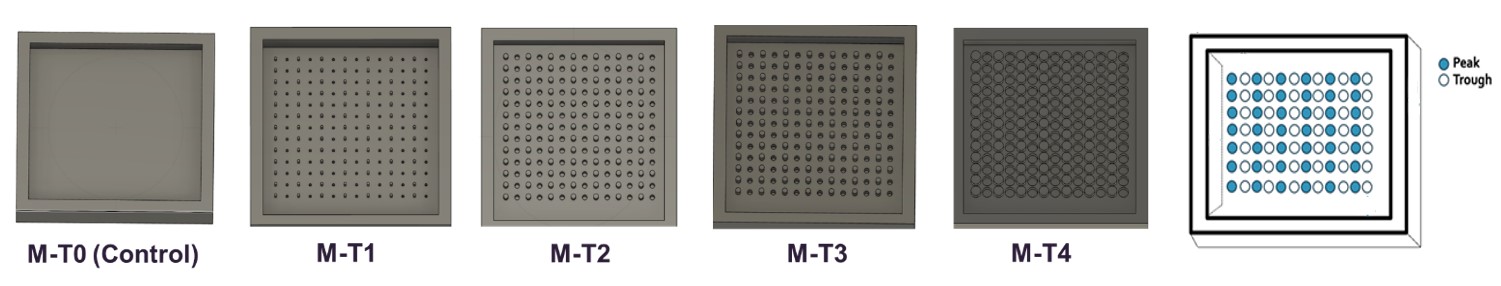 Figure 1. 3D Auto-CAD images of the designed molds for casting substrates with topographical levels from low to high including M-T0 (control level, no peak or trough), M-T1 (level 1), M-T2 (level 2), M-T3 (level 3), M-T4 (level 4).
Figure 1. 3D Auto-CAD images of the designed molds for casting substrates with topographical levels from low to high including M-T0 (control level, no peak or trough), M-T1 (level 1), M-T2 (level 2), M-T3 (level 3), M-T4 (level 4).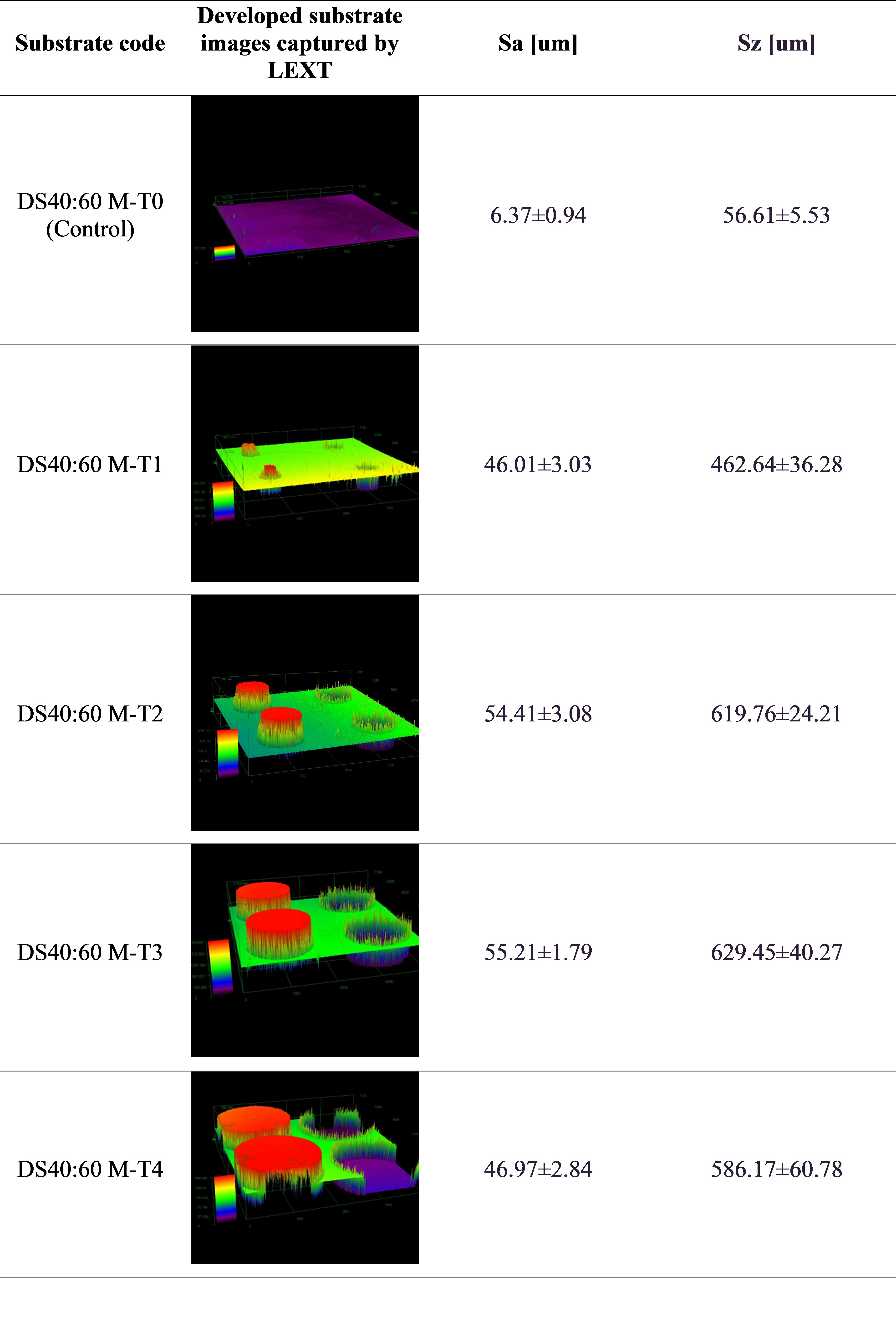 Figure 2. Microscopic LEXT images of developed DS substrates incorporated with topographical levels (M-T0 to M-T4) and measured topographical parameters (n=3), shown as mean value ± standard deviation (SD), including Sa (arithmetic mean of height) and Sz (sum of maximum peak height and maximum valley depth) of the developed DS substrates.
Figure 2. Microscopic LEXT images of developed DS substrates incorporated with topographical levels (M-T0 to M-T4) and measured topographical parameters (n=3), shown as mean value ± standard deviation (SD), including Sa (arithmetic mean of height) and Sz (sum of maximum peak height and maximum valley depth) of the developed DS substrates.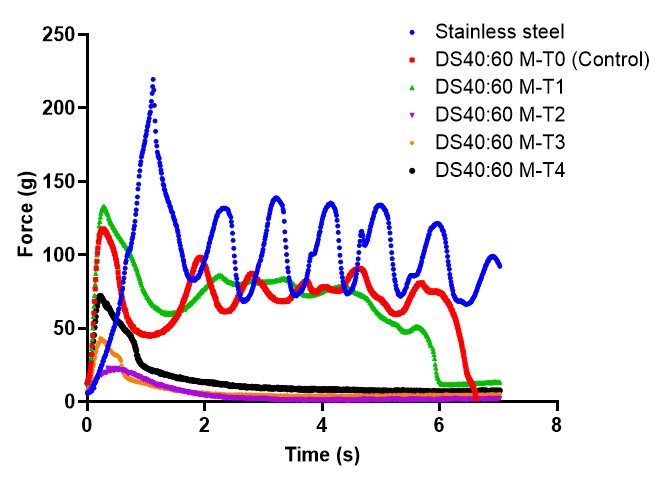 Figure 3. Mean peel adhesion profiles of an over the counter (OTC) TDS product (NicoDerm) on stainless steel and on 5 developed DS substrates incorporated with topographical levels (M-T0 to M-T4, low to high) (n=2).
Figure 3. Mean peel adhesion profiles of an over the counter (OTC) TDS product (NicoDerm) on stainless steel and on 5 developed DS substrates incorporated with topographical levels (M-T0 to M-T4, low to high) (n=2).