Manufacturing and Analytical Characterization - Chemical
(T1530-02-12) Development of Spray Freeze Drying as a Novel Strategy to Enhance the Oral Delivery of Proteins/Peptides
Tuesday, November 11, 2025
3:30 PM - 4:30 PM CT
- RK
Radha Kulkarni, MS (she/her/hers)
Graduate Student
University of Connecticut
Storrs, Connecticut, United States - RK
Radha Kulkarni, MS (she/her/hers)
Graduate Student
University of Connecticut
Storrs, Connecticut, United States - PP
Pawan Kumar Pandey, MS
Graduate Student
University of Connecticut
Storrs, Connecticut, United States 
Diane Burgess, PhD
Board of Trustees Distinguished Professor of Pharmaceutics Pfizer Distinguished Chair of Pharmaceuti
University of Connecticut
Storrs, Connecticut, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Development of oral delivery systems for macromolecules is highly challenging due to their susceptibility to degradation at low stomach pH, as well as impaired intestinal absorption as a result of their large molecular size. Mucoadhesive microparticles delivered via enteric coated capsules overcome these challenges by selectively targeting the intestinal epithelium, where they increase peptide residence time, and facilitate systemic absorption, thereby eliminating the need for toxic permeation enhancers. Spray freeze drying overcomes the limitations of conventional drying technologies such as spray drying and freeze drying due to its lower processing time and lower processing temperature. This study aims to: i) investigate spray freeze drying as a reliable technique to formulate mucoadhesive microparticles for oral delivery of peptides; ii) understand the impact of excipients on the critical quality attributes (CQAs) of spray freeze dried microparticles; and iii) perform a comparison between spray drying, freeze drying, and spray freeze drying as a prospective technique for mucoadhesive oral delivery dosage forms.
Methods: Spray freeze drying was performed in house using a specially designed cryogenic vessel and an optimized method. The spray solution model peptide (octreotide acetate), mucoadhesive polymer (sodium alginate), and excipients (a combination of bulking agent and stabilizers) was sprayed into the cryogenic vessel containing liquid nitrogen via a twin fluid nozzle (Fig. 1A and B). The frozen particles were transferred into a benchtop freeze dryer (shelf temperature -40 °C), and freeze dried at 40 °C, 0.1 mbar for 4 h (primary drying) and at 25 °C (ramp rate of 0.2 °C/min) for 3 h at 0.1 mbar (secondary drying). The initial formulation optimization involved investigation of the impact of different formulation compositions on the CQAs of spray freeze dried microparticles (flow properties, DSC thermograms, and FTIR). The physicochemical characteristics of the optimized spray freeze dried formulation and of formulations prepared viaconventional drying techniques (spray drying and freeze drying) were compared using DSC, FTIR, and second derivative FTIR. In vitro drug release from the formulations was performed after filling into enteric coated capsules and placing in USP I apparatus. Formulation morphology was visualized via scanning electron microscopy. As a proof of concept, an ex vivo mucoadhesion study to understand intestinal retention was performed on freshly excised porcine duodenum. The optimized spray freeze dried formulation was filled onto the intestinal tissue (the ends of the tissue were attached to silicon tubing). Phosphate buffer pH 6.8 (0.2 mL/min) was passed through the tissue and the peptide concentration in the eluent was collected and analysed every 10 mins via HPLC.
Results: The comprehensive investigation utilizing different combinations of excipients revealed that slight differences in formulation compositions can have a profound impact on the physicochemical characteristics of peptide loaded spray freeze dried microparticles. It was observed that formulations containing bulking agents such as mannitol and phenylalanine (which crystallized during freeze drying) showed better flow (i.e., lower Hausner ratio). This is probably a result of their improved mechanical structure (Fig. 1C and D). The FTIR spectra showed peak broadening at ~3500 cm-1suggesting interactions between the OH groups of the lyoprotectants and the peptide. Peaks at 1700 cm-1 and 2900 cm-1 were observed for all the formulations, which corresponded to the C=O and O-H groups of the peptide, respectively (Fig. 1E). The formulation which showed good particle flow and peptide stability was selected for further studies. Interestingly, DSC analysis showed that spray dried microparticles had an amorphous structure (peptide and excipients) (Fig. 2A). There were no observed differences in the FTIR spectra for the three drying techniques (Fig. 2B). This may be attributed to the higher stability of the peptide against shear and temperature stress. The in vitro peptide release behaviour of the formulations corelated with formulation morphology. The freeze dried formulation showed faster drug release due to the higher porosity of the lyophilized cake. Conversely, the spray dried and spray freeze dried formulations showed slower release with similar release profiles. This may be due to compensation between the higher particle size (~60 µm) and higher porosity of the spray freeze dried microparticles compared to the lower particle size (~2 µm) and lower porosity of the spray dried microparticles (Fig. 2C and D). Finally, the ex vivo mucoadhesion study showed longer retention of the peptide for the mucoadhesive spray freeze dried microparticles compared to raw API, which signifies higher drug residence time for the peptide in the intestine (Fig. 3).
Conclusion: This research introduces spray freeze drying as an effective alternative drying technology for oral delivery of challenging molecules. It was demonstrated that mucoadhesive spray freeze dried microparticles increased the residence time of the peptide in ex vivo porcine tissue studies while maintaining peptide stability. Different excipients (i.e., bulking agents and lyoprotectants) can have a profound impact on the physicochemical characteristics of the formulations and should be considered during formulation optimization and selection.
References: Tingting Li et.al; 'Pectin micro-particles for peptide delivery: Optimization of spray drying processing'; International Journal of Pharmaceutics; 2022 Feb 5:613:121384. doi: 10.1016/j.ijpharm.2021.121384.
Acknowledgements: This work was supported by the University of Connecticut and the Dane O. Kildsig Center for Pharmaceutical Processing and Research (CPPR).
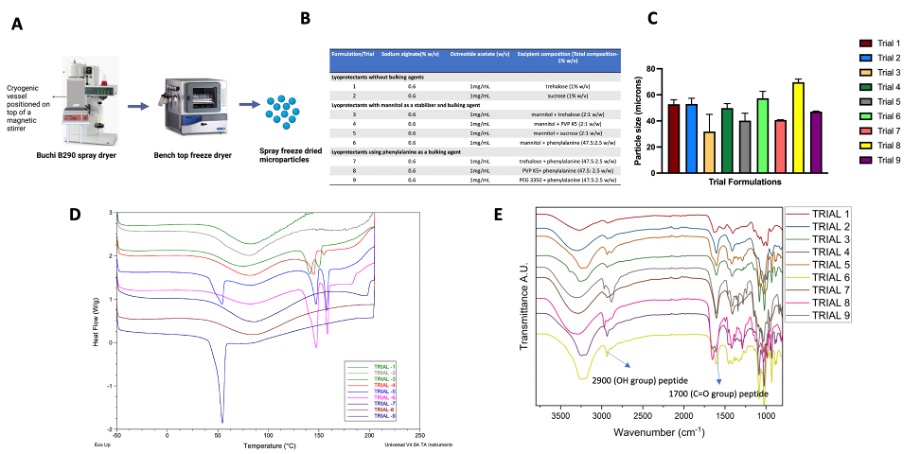 Fig. 1. A) Ilustration of the spray freeze drying process developed in-house; B) compositions of the initial optimization trial formulations comprising of different combinations of bulking agents and lyoprotectants; C) Hausner ratio for different trial formulations, values above 1.4 are considered as poorly flowing powders; D) DSC thermograms of trial formulations with characteristic melting peaks of drug and excipients; and E) FTIR spectra of the trial formulations.
Fig. 1. A) Ilustration of the spray freeze drying process developed in-house; B) compositions of the initial optimization trial formulations comprising of different combinations of bulking agents and lyoprotectants; C) Hausner ratio for different trial formulations, values above 1.4 are considered as poorly flowing powders; D) DSC thermograms of trial formulations with characteristic melting peaks of drug and excipients; and E) FTIR spectra of the trial formulations.
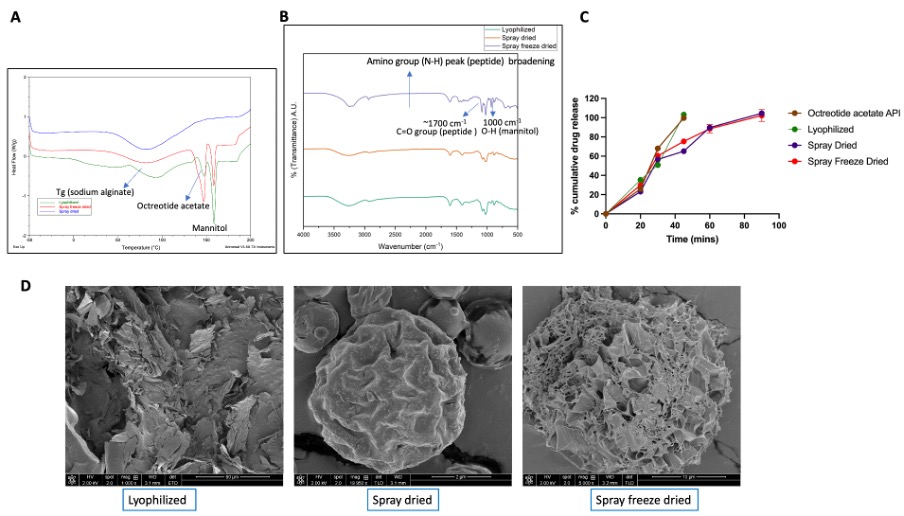 Fig. 2 A) DSC thermograms of formulations prepared using different techniques (i.e, spray drying freeze drying, and spray freeze drying/lyophilization); B) FTIR spectra of the formulations prepared using different drying techniques; C) In vitro release of the formulations prepared using different drying techniques via USP 1 apparatus with phosphate buffer (pH 6.8) (150 mL, 37 °C, 25 RPM) (mean ±SD, n=3); and D) SEM micrographs of the formulations prepared using different drying techniques.
Fig. 2 A) DSC thermograms of formulations prepared using different techniques (i.e, spray drying freeze drying, and spray freeze drying/lyophilization); B) FTIR spectra of the formulations prepared using different drying techniques; C) In vitro release of the formulations prepared using different drying techniques via USP 1 apparatus with phosphate buffer (pH 6.8) (150 mL, 37 °C, 25 RPM) (mean ±SD, n=3); and D) SEM micrographs of the formulations prepared using different drying techniques.
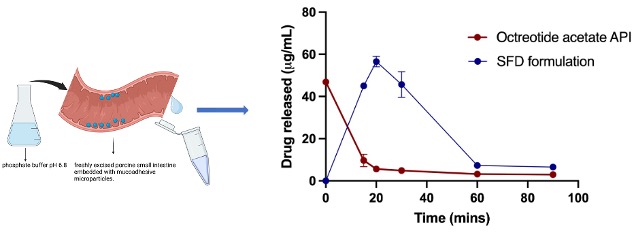 Fig. 3. Ex vivo mucoadhesion/intestinal retention study using excised porcine intestine (duodenum) (mean ±SD, n=3).
Fig. 3. Ex vivo mucoadhesion/intestinal retention study using excised porcine intestine (duodenum) (mean ±SD, n=3).
Methods: Spray freeze drying was performed in house using a specially designed cryogenic vessel and an optimized method. The spray solution model peptide (octreotide acetate), mucoadhesive polymer (sodium alginate), and excipients (a combination of bulking agent and stabilizers) was sprayed into the cryogenic vessel containing liquid nitrogen via a twin fluid nozzle (Fig. 1A and B). The frozen particles were transferred into a benchtop freeze dryer (shelf temperature -40 °C), and freeze dried at 40 °C, 0.1 mbar for 4 h (primary drying) and at 25 °C (ramp rate of 0.2 °C/min) for 3 h at 0.1 mbar (secondary drying). The initial formulation optimization involved investigation of the impact of different formulation compositions on the CQAs of spray freeze dried microparticles (flow properties, DSC thermograms, and FTIR). The physicochemical characteristics of the optimized spray freeze dried formulation and of formulations prepared viaconventional drying techniques (spray drying and freeze drying) were compared using DSC, FTIR, and second derivative FTIR. In vitro drug release from the formulations was performed after filling into enteric coated capsules and placing in USP I apparatus. Formulation morphology was visualized via scanning electron microscopy. As a proof of concept, an ex vivo mucoadhesion study to understand intestinal retention was performed on freshly excised porcine duodenum. The optimized spray freeze dried formulation was filled onto the intestinal tissue (the ends of the tissue were attached to silicon tubing). Phosphate buffer pH 6.8 (0.2 mL/min) was passed through the tissue and the peptide concentration in the eluent was collected and analysed every 10 mins via HPLC.
Results: The comprehensive investigation utilizing different combinations of excipients revealed that slight differences in formulation compositions can have a profound impact on the physicochemical characteristics of peptide loaded spray freeze dried microparticles. It was observed that formulations containing bulking agents such as mannitol and phenylalanine (which crystallized during freeze drying) showed better flow (i.e., lower Hausner ratio). This is probably a result of their improved mechanical structure (Fig. 1C and D). The FTIR spectra showed peak broadening at ~3500 cm-1suggesting interactions between the OH groups of the lyoprotectants and the peptide. Peaks at 1700 cm-1 and 2900 cm-1 were observed for all the formulations, which corresponded to the C=O and O-H groups of the peptide, respectively (Fig. 1E). The formulation which showed good particle flow and peptide stability was selected for further studies. Interestingly, DSC analysis showed that spray dried microparticles had an amorphous structure (peptide and excipients) (Fig. 2A). There were no observed differences in the FTIR spectra for the three drying techniques (Fig. 2B). This may be attributed to the higher stability of the peptide against shear and temperature stress. The in vitro peptide release behaviour of the formulations corelated with formulation morphology. The freeze dried formulation showed faster drug release due to the higher porosity of the lyophilized cake. Conversely, the spray dried and spray freeze dried formulations showed slower release with similar release profiles. This may be due to compensation between the higher particle size (~60 µm) and higher porosity of the spray freeze dried microparticles compared to the lower particle size (~2 µm) and lower porosity of the spray dried microparticles (Fig. 2C and D). Finally, the ex vivo mucoadhesion study showed longer retention of the peptide for the mucoadhesive spray freeze dried microparticles compared to raw API, which signifies higher drug residence time for the peptide in the intestine (Fig. 3).
Conclusion: This research introduces spray freeze drying as an effective alternative drying technology for oral delivery of challenging molecules. It was demonstrated that mucoadhesive spray freeze dried microparticles increased the residence time of the peptide in ex vivo porcine tissue studies while maintaining peptide stability. Different excipients (i.e., bulking agents and lyoprotectants) can have a profound impact on the physicochemical characteristics of the formulations and should be considered during formulation optimization and selection.
References: Tingting Li et.al; 'Pectin micro-particles for peptide delivery: Optimization of spray drying processing'; International Journal of Pharmaceutics; 2022 Feb 5:613:121384. doi: 10.1016/j.ijpharm.2021.121384.
Acknowledgements: This work was supported by the University of Connecticut and the Dane O. Kildsig Center for Pharmaceutical Processing and Research (CPPR).
 Fig. 1. A) Ilustration of the spray freeze drying process developed in-house; B) compositions of the initial optimization trial formulations comprising of different combinations of bulking agents and lyoprotectants; C) Hausner ratio for different trial formulations, values above 1.4 are considered as poorly flowing powders; D) DSC thermograms of trial formulations with characteristic melting peaks of drug and excipients; and E) FTIR spectra of the trial formulations.
Fig. 1. A) Ilustration of the spray freeze drying process developed in-house; B) compositions of the initial optimization trial formulations comprising of different combinations of bulking agents and lyoprotectants; C) Hausner ratio for different trial formulations, values above 1.4 are considered as poorly flowing powders; D) DSC thermograms of trial formulations with characteristic melting peaks of drug and excipients; and E) FTIR spectra of the trial formulations.  Fig. 2 A) DSC thermograms of formulations prepared using different techniques (i.e, spray drying freeze drying, and spray freeze drying/lyophilization); B) FTIR spectra of the formulations prepared using different drying techniques; C) In vitro release of the formulations prepared using different drying techniques via USP 1 apparatus with phosphate buffer (pH 6.8) (150 mL, 37 °C, 25 RPM) (mean ±SD, n=3); and D) SEM micrographs of the formulations prepared using different drying techniques.
Fig. 2 A) DSC thermograms of formulations prepared using different techniques (i.e, spray drying freeze drying, and spray freeze drying/lyophilization); B) FTIR spectra of the formulations prepared using different drying techniques; C) In vitro release of the formulations prepared using different drying techniques via USP 1 apparatus with phosphate buffer (pH 6.8) (150 mL, 37 °C, 25 RPM) (mean ±SD, n=3); and D) SEM micrographs of the formulations prepared using different drying techniques. Fig. 3. Ex vivo mucoadhesion/intestinal retention study using excised porcine intestine (duodenum) (mean ±SD, n=3).
Fig. 3. Ex vivo mucoadhesion/intestinal retention study using excised porcine intestine (duodenum) (mean ±SD, n=3). 