Formulation and Delivery - Chemical
(T1530-08-54) Modulating Precipitation of Small Molecule Drugs upon Intraperitoneal Injection Using Cyclodextrin Containing Formulations
- JT
Jack Terry, MS (he/him/his)
Graduate Research Assistant
University of Kansas
Lawrence, Kansas, United States - JT
Jack Terry, MS (he/him/his)
Graduate Research Assistant
University of Kansas
Lawrence, Kansas, United States - JB
Josephine Banks, BS
Graduate Student
Purdue University
West Layfayette, Indiana, United States - JH
Josephine Herrold
Undergraduate student
University of Kansas
Lawrence, Kansas, United States - MF
Mei Feng, PhD
Associate Researcher
University of Kansas
Lawrence, Kansas, United States - IW
Indika Warnakula, Ph.D.
Postdoctoral Research Scientist
University of Kansas
Lawrence, Kansas, United States - ST
Sara Thomas, Ph.D.
Researcher
University of Kansas
Lawrence, Kansas, United States 
Michael J. Hageman, Ph.D.
Valentino J. Stella Distinguished Professor, Pharmaceutical Chemistry
University of Kansas
Lawrence, Kansas, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: Drug formulations were prepared by dissolving celecoxib into neat cosolvent (Polyethylene Glycol 300 (PEG300)). Upon complete dissolution formulations were diluted with aqueous solutions of Captisol (Sulfobutylether beta-cyclodextrin sodium salt) ranging from 0-50% (w/v). Final cosolvent concentrations ranged from 10-70%. The solubility of celecoxib was measured in a matrix of these formulations using both a shake flask method and a precipitation induction method. The shake flask method was conducted via addition of excess crystalline celecoxib solid to each formulation. Analysis was conducted by centrifuging, sampling supernatant, diluting in LC gradient, and finally injection into a Waters Acquity UPLC system. Absorbance via a UV flow cell was measured as a means of quantifying celecoxib drug concentration. This sampling method was repeated until equivalent concentration values were measured over a 24-hour period. The precipitation method was much the same but samples were prepared via addition of high concentration PEG stock to the associated formulation diluents rather than addition of solid crystalline drug. Sampling practices were identical following formulation preparation for this method. Drug formulations were diluted between 10-100x into water and stirred to mimic the dilution possible following intraperitoneal injection. Following dilution, occlusion of visible light was measured using a UV dip probe measuring absorbance at 450 nm. This allowed for the real time quantification of precipitation upon dilution. Precipitation was measured for 15 minutes. To quantify permeability of model formulations and more specifically presence of free drug in solution, in vitro microdialysis was utilized. Drug formulations were diluted in the same manner that was utilized in UV dip probe experiments above. A linear CMA 30 probe was primed with saline and submerged into diluent. The probe was pumped with saline at 1 uL/min and samples were collected at 15 minute time intervals following initial formulation dilution. Samples were analyzed using LCMS.
Results: The solubility of Celecoxib increased linearly in aqueous solutions containing Captisol as the sole excipient. Solubility increased logarithmically in solutions containing PEG as the sole excipient. A 3-dimensional surface was generated from measuring solubility of Celecoxib in formulations containing both model excipients. This data indicated that the solubility enhancement from addition of Captisol to the aqueous diluent in cosolvent PEG formulations was relatively insignificant and the main factor was the concentration of PEG in this case. The solubility in formulations containing both excipient species were not significantly different when measured by the shake flask or precipitation method. The addition of Captisol seems to have a meaningful effect on the speed of precipitation observed via UV dip probe. Additionally, Captisol seems to reduce the ability of celecoxib to permeate across the membrane present in CMA 30 probes (6 kDa cut-off).
Conclusion: Enhancement in solubility from the addition of both excipient species was as expected. The observed enhancement from varying levels of Captisol indicates 1:1 binding with celecoxib as expected. The similarity in result from the shake flask and precipitation methods indicate that the solid species observed precipitating from supersaturated solution of celecoxib likely is exhibiting the same crystalline habit of the commercially available solid form. The results of the UV dip probe experiment and the in vitro microdialysis experiment indicate that presence of Captisol has the potential to modulate the precipitation of celecoxib upon dilution but may reduce the flux rate of the compound due to complexation with Captisol. To determine how this effect will change the bioavailability of celecoxib (more broadly BCS class II compound which can complex with cyclodextrins) formulations with varying Captisol levels will by administered intraperitoneally to rats.
References: Al Shoyaib, A., Archie, S. R., & Karamyan, V. T. (2019). Intraperitoneal Route of Drug Administration: Should it Be Used in Experimental Animal Studies? Pharm Res, 37(1), 12. https://doi.org/10.1007/s11095-019-2745-x
Beier, H., Kaiser, K., Langhans, M., Malmendier, K., Sluijsmans, I., & Weiher, J. (2007). Peritoneal microdialysis in freely moving rodents: an alternative to blood sampling for pharmacokinetic studies in the rat and the mouse. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences, 30(1), 75–83. https://doi.org/10.1016/j.ejps.2006.10.005
A, J. (2008). Review of the cosolvency models for predicting solubility of drugs in water-cosolvent mixtures. Journal of Pharmaceutical Sciences, 11(1), 32-58. https://doi.org/10.18433/j3pp4k
Stella VJ, H. Q. (2008). Cyclodextrins. Toxicologic pathology, 36(1), 30-42. https://doi.org/10.1177/0192623307310945
Acknowledgements: We would like to acknowledge the support of Ligand Pharmaceuticals and the Stella Endowment fund for this project
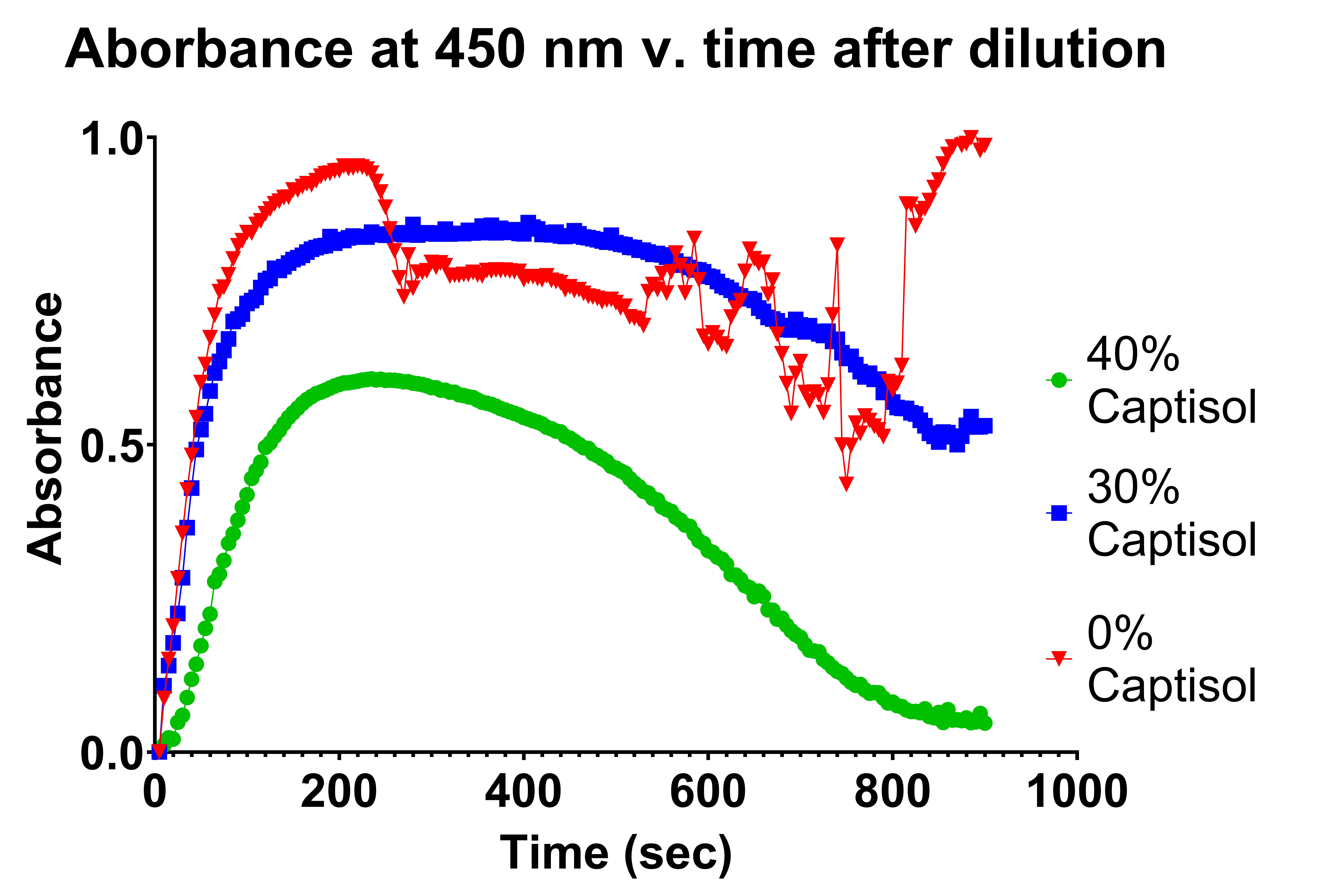 Figure 1-Visible light absorption of varying formulations of celecoxib upon dilution. 15 mg/mL celecoxib in 7:3 PEG:aqueous diluted 100x. Legend indicates identity of aqueous component of formulation in w/v
Figure 1-Visible light absorption of varying formulations of celecoxib upon dilution. 15 mg/mL celecoxib in 7:3 PEG:aqueous diluted 100x. Legend indicates identity of aqueous component of formulation in w/v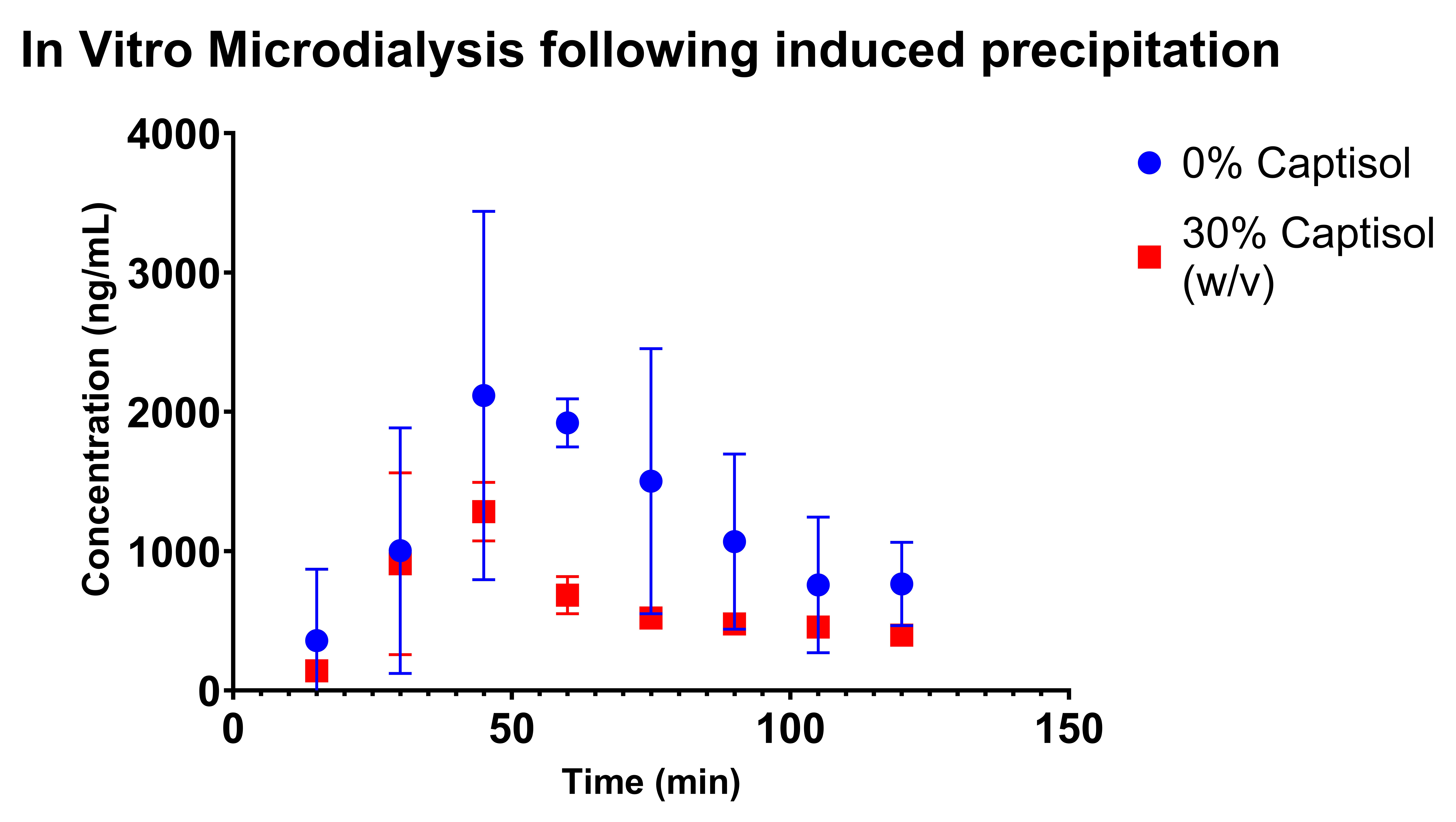 Figure 2-Measured concentrations of in vitro microdialysis samples collected following 100x dilution of 7:3
Figure 2-Measured concentrations of in vitro microdialysis samples collected following 100x dilution of 7:3 PEG:aqueous. Original formulation concentrations were 15 mg/mL celecoxib. Aqueous component of formulation is indicated in legend
