Formulation and Delivery - Biomolecular
(W0930-09-55) Design of Site-Specific Enteric Capsule for Terminal Ileal Drug Delivery
Wednesday, November 12, 2025
9:30 AM - 10:30 AM CT
- EM
Elisa Millet, MS
Jr. Scientist
Capsugel France SAS / Lonza
Colmar, Alsace, France - EM
Elisa Millet, MS
Jr. Scientist
Capsugel France SAS / Lonza
Colmar, Alsace, France 
Vincent Jannin, PhD (he/him/his)
Director, R&D
Capsugel France SAS / Lonza
Colmar, Alsace, France- JO
Joseph P. O'Shea, Ph.D.
Lecturer in Pharmaceutics
University College Cork
Cork City, Cork, Ireland 
Brendan T. Griffin, Ph.D.
Professor in Biopharmaceutics & Drug Delivery; Head of School of Pharmacy
University College Cork
Cork City, Cork, Ireland
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Capsule-based drug delivery has evolved significantly, offering improved protection of active pharmaceutical ingredients (APIs) and enabling targeted release within the gastrointestinal (GI) tract. Innovations in polymer science have expanded capsule functionality, particularly for delivering labile molecules such as peptides, proteins, and RNA-based therapeutics (Nirmal et al., 2024). Due to their inherent instability in the GI environment, RNA-based drugs require protective oral delivery systems (Zhang et al., 2021). Enteric capsules provide this protection by resisting gastric conditions and releasing their contents at specific intestinal sites. This study aims to develop a dual-layer enteric capsule designed for site-specific release at the end of the ileum, with the potential to surpass the performance of commercial Enprotect® capsules (Grimm et al., 2023; Rump et al., 2022). The system comprises: an inner water-soluble layer of hydroxypropyl methylcellulose (HPMC) blended with pullulan, and an outer ethylcellulose layer incorporating sodium alginate as a pore-forming agent The primary objective is to evaluate the impact of sodium alginate concentration (3%, 4%, 9%) and curing conditions (temperature/relative humidity) on capsule disintegration behavior across physiologically relevant pH stages.
Methods: Size 1 hard gelatin capsules were filled with 0.4 g of lactose (Pharmatose 200M) containing 0.1% red carrot extract, which served as a visual marker for disintegration. Capsules were coated with alginate solutions at three concentrations (3%, 4%, and 9%) and subjected to a matrix of curing conditions, including four temperatures (40 °C, 60 °C, 70 °C, and 80 °C) and four levels of relative humidity (5% RH [dry], 35%, 40%, and 75%). Each formulation and curing condition (n = 6 per group) was evaluated using a SOTAX DT2 disintegration tester, following the three-stage protocol outlined in the European Pharmacopoeia for gastro-resistant dosage forms. Capsules were first exposed to 0.1 M HCl for 2 hours (gastric phase), followed by 1 hour in phosphate buffer at pH 6.8, and finally 1 hour in phosphate buffer at pH 7.4. Disintegration discs were used during the final stage. Disintegration was defined as complete rupture of the capsule shell and visible release of the colored payload. Scanning Electron Microscopy (SEM) was performed on cross-sections of selected capsules using a FlexSEM 1000 HITACHI (SU1000) operating at 10 kV to assess film morphology and structural integrity.
Results: All capsule formulations demonstrated resistance to the acidic phase, confirming effective protection against gastric conditions. At pH 6.8, capsules containing 9% alginate disintegrated prematurely (within 30 minutes), regardless of curing parameters, suggesting high porosity and inadequate structural integrity for targeted ileal delivery. Capsules formulated with 3% alginate exhibited considerable variability; in particular, those cured at 70 °C and 75% relative humidity disintegrated at 00:48:24 ± 00:23:12, indicating suboptimal mechanical strength. In contrast, capsules with 4% alginate remained intact at pH 6.8 under all curing conditions and successfully progressed to the final pH stage. At pH 7.4, the majority of 4% alginate capsules disintegrated within 30 minutes. Notably, the capsule cured at 70 °C and 40% RH exhibited delayed disintegration (01:00:00 ± 00:00:00), suggesting enhanced resistance and improved suitability for terminal ileum targeting. Scanning electron microscopy (SEM) of capsule cross-sections revealed distinct morphological differences in the outer layers. Curing at 70 °C under dry conditions produced a loosely packed, stratified structure, indicative of weak polymer interactions and poor cohesion. In contrast, curing at 70 °C and 40% RH resulted in a smooth, dense, and continuous bilayer with low porosity, reflecting enhanced crosslinking and improved mechanical integrity. Curing at 70 °C and 75% RH produced a highly porous and irregular film with evident delamination, likely due to excessive alginate swelling during the curing process.
Conclusion: Capsule integrity and disintegration behavior are strongly influenced by both alginate concentration and curing conditions. Formulations containing 3% alginate exhibited poor mechanical stability and inconsistent performance, while those with 9% alginate displayed excessive porosity, undermining their resistance in enteric conditions. Capsules formulated with 4% alginate and cured at 70 °C with 40% relative humidity demonstrated optimal performance, forming a mechanically robust bilayer structure capable of supporting targeted release at the distal ileum. These findings were corroborated by SEM analysis, which revealed a compact, homogenous outer film under these specific curing conditions. Further optimization is necessary to enhance film uniformity and batch-to-batch reproducibility. Future efforts will focus on refining alginate content and curing parameters to reliably produce capsules suitable for site-specific oral delivery of sensitive biologics.
References: Nirmal, P., Selvi, G., Vignesh, S., Kumar, P., 2024. Current Progressions in Dual Drug Delivery: Exploring Tablet-In-Capsule Technology 30.
Zhang, S., Wei, F., Han, X., 2018. Antibacterial edible film of sodium alginate/pullulan incorporated with capsaicin. New Journal of Chemistry 42.
Rump, A., Kromrey, M.L., Scheuch, E., Jannin, V., Rehenbrock, L., Tzvetkov, M., Weitschies, W., Grimm, M. 2022 In vivo evaluation of a gastro-resistant HPMC-based “Next Generation Enteric” capsule. Pharmaceutics. 14 1999
Grimm, M., Rump, A., Kromrey, M.L., Morof, F., Dumont, C., Jannin, V., Tzvetkov, M.V., Weitschies, W. 2023 In vivo evaluation of a gastro-resistant Enprotect® capsule under postprandial conditions. Pharmaceutics. 15 2576.
Acknowledgements: Funding for this publication is provided under the GENEGUT (https://genegut.eu/) project funded by the European Commission as a Horizon Europe Research and Innovation Action (RIA) under the call tools and technologies for a healthy society (2021) (HORIZON-HLTH-2021-TOOL-06, GA 101057491).
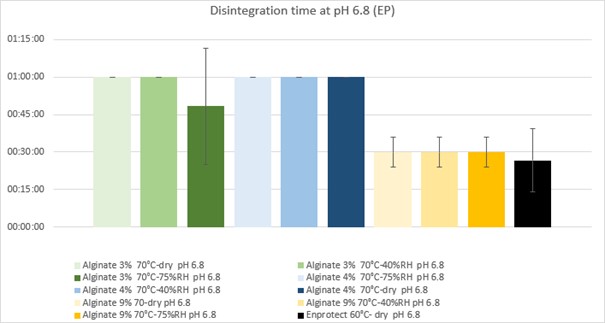 Figure 1 – Disintegration time at pH 6.8: Average disintegration time (± standard deviation) for capsules with 3%, 4%, and 9% sodium alginate cured under different conditions.
Figure 1 – Disintegration time at pH 6.8: Average disintegration time (± standard deviation) for capsules with 3%, 4%, and 9% sodium alginate cured under different conditions.
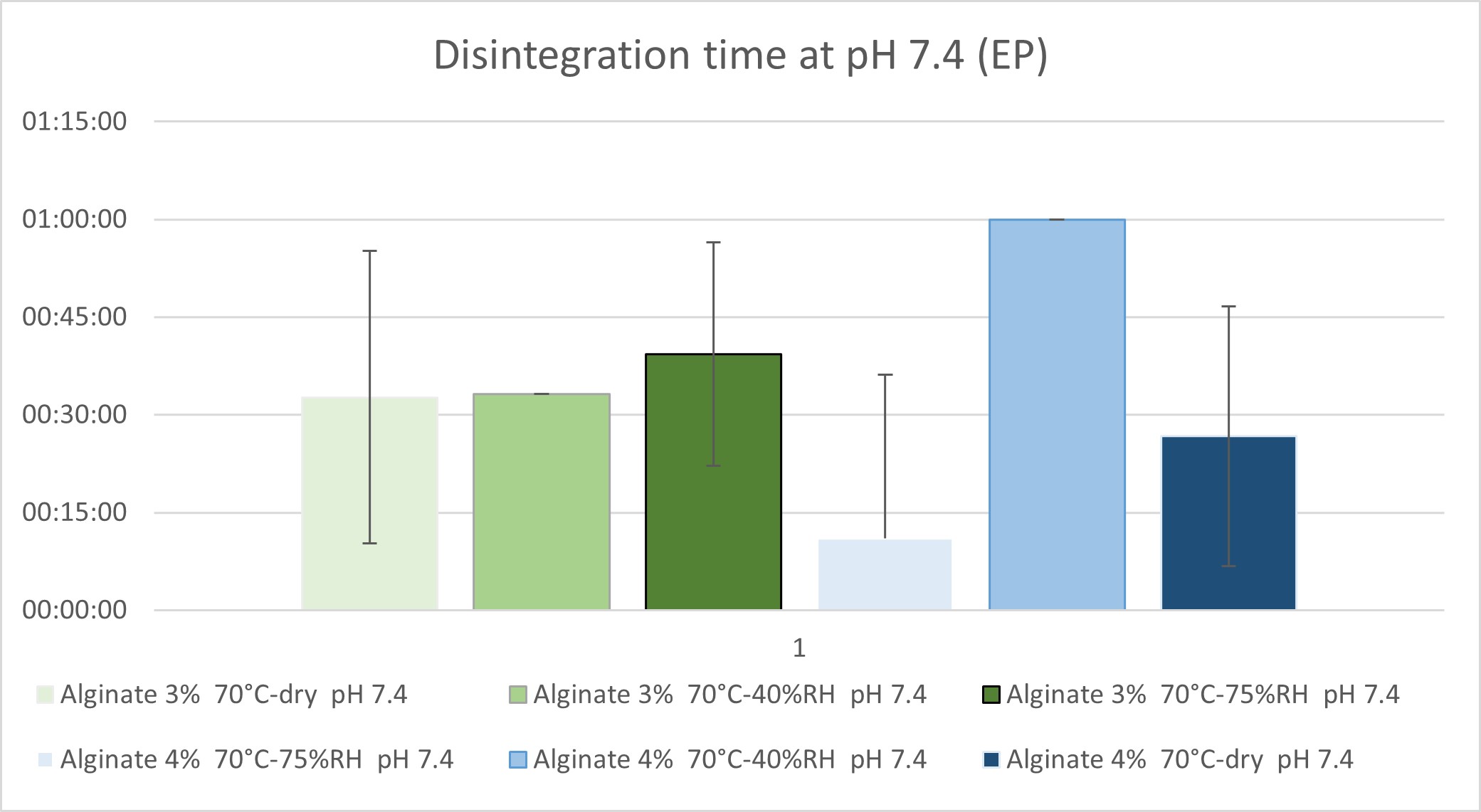 Figure 2 – Disintegration time at pH 7.4: Time to complete capsule opening at pH 7.4 for all samples that resisted pH 6.8.
Figure 2 – Disintegration time at pH 7.4: Time to complete capsule opening at pH 7.4 for all samples that resisted pH 6.8.
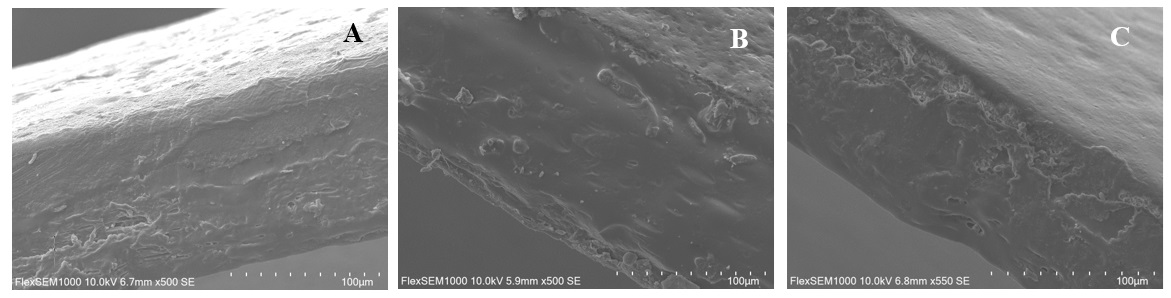 Figure 3 — SEM analysis of capsule shell cross-sections after curing at 70°C with different relative humidities. A: 70°C – Dry; B: 70°C – 40% RH; C: 70°C – 75% RH
Figure 3 — SEM analysis of capsule shell cross-sections after curing at 70°C with different relative humidities. A: 70°C – Dry; B: 70°C – 40% RH; C: 70°C – 75% RH
Methods: Size 1 hard gelatin capsules were filled with 0.4 g of lactose (Pharmatose 200M) containing 0.1% red carrot extract, which served as a visual marker for disintegration. Capsules were coated with alginate solutions at three concentrations (3%, 4%, and 9%) and subjected to a matrix of curing conditions, including four temperatures (40 °C, 60 °C, 70 °C, and 80 °C) and four levels of relative humidity (5% RH [dry], 35%, 40%, and 75%). Each formulation and curing condition (n = 6 per group) was evaluated using a SOTAX DT2 disintegration tester, following the three-stage protocol outlined in the European Pharmacopoeia for gastro-resistant dosage forms. Capsules were first exposed to 0.1 M HCl for 2 hours (gastric phase), followed by 1 hour in phosphate buffer at pH 6.8, and finally 1 hour in phosphate buffer at pH 7.4. Disintegration discs were used during the final stage. Disintegration was defined as complete rupture of the capsule shell and visible release of the colored payload. Scanning Electron Microscopy (SEM) was performed on cross-sections of selected capsules using a FlexSEM 1000 HITACHI (SU1000) operating at 10 kV to assess film morphology and structural integrity.
Results: All capsule formulations demonstrated resistance to the acidic phase, confirming effective protection against gastric conditions. At pH 6.8, capsules containing 9% alginate disintegrated prematurely (within 30 minutes), regardless of curing parameters, suggesting high porosity and inadequate structural integrity for targeted ileal delivery. Capsules formulated with 3% alginate exhibited considerable variability; in particular, those cured at 70 °C and 75% relative humidity disintegrated at 00:48:24 ± 00:23:12, indicating suboptimal mechanical strength. In contrast, capsules with 4% alginate remained intact at pH 6.8 under all curing conditions and successfully progressed to the final pH stage. At pH 7.4, the majority of 4% alginate capsules disintegrated within 30 minutes. Notably, the capsule cured at 70 °C and 40% RH exhibited delayed disintegration (01:00:00 ± 00:00:00), suggesting enhanced resistance and improved suitability for terminal ileum targeting. Scanning electron microscopy (SEM) of capsule cross-sections revealed distinct morphological differences in the outer layers. Curing at 70 °C under dry conditions produced a loosely packed, stratified structure, indicative of weak polymer interactions and poor cohesion. In contrast, curing at 70 °C and 40% RH resulted in a smooth, dense, and continuous bilayer with low porosity, reflecting enhanced crosslinking and improved mechanical integrity. Curing at 70 °C and 75% RH produced a highly porous and irregular film with evident delamination, likely due to excessive alginate swelling during the curing process.
Conclusion: Capsule integrity and disintegration behavior are strongly influenced by both alginate concentration and curing conditions. Formulations containing 3% alginate exhibited poor mechanical stability and inconsistent performance, while those with 9% alginate displayed excessive porosity, undermining their resistance in enteric conditions. Capsules formulated with 4% alginate and cured at 70 °C with 40% relative humidity demonstrated optimal performance, forming a mechanically robust bilayer structure capable of supporting targeted release at the distal ileum. These findings were corroborated by SEM analysis, which revealed a compact, homogenous outer film under these specific curing conditions. Further optimization is necessary to enhance film uniformity and batch-to-batch reproducibility. Future efforts will focus on refining alginate content and curing parameters to reliably produce capsules suitable for site-specific oral delivery of sensitive biologics.
References: Nirmal, P., Selvi, G., Vignesh, S., Kumar, P., 2024. Current Progressions in Dual Drug Delivery: Exploring Tablet-In-Capsule Technology 30.
Zhang, S., Wei, F., Han, X., 2018. Antibacterial edible film of sodium alginate/pullulan incorporated with capsaicin. New Journal of Chemistry 42.
Rump, A., Kromrey, M.L., Scheuch, E., Jannin, V., Rehenbrock, L., Tzvetkov, M., Weitschies, W., Grimm, M. 2022 In vivo evaluation of a gastro-resistant HPMC-based “Next Generation Enteric” capsule. Pharmaceutics. 14 1999
Grimm, M., Rump, A., Kromrey, M.L., Morof, F., Dumont, C., Jannin, V., Tzvetkov, M.V., Weitschies, W. 2023 In vivo evaluation of a gastro-resistant Enprotect® capsule under postprandial conditions. Pharmaceutics. 15 2576.
Acknowledgements: Funding for this publication is provided under the GENEGUT (https://genegut.eu/) project funded by the European Commission as a Horizon Europe Research and Innovation Action (RIA) under the call tools and technologies for a healthy society (2021) (HORIZON-HLTH-2021-TOOL-06, GA 101057491).
 Figure 1 – Disintegration time at pH 6.8: Average disintegration time (± standard deviation) for capsules with 3%, 4%, and 9% sodium alginate cured under different conditions.
Figure 1 – Disintegration time at pH 6.8: Average disintegration time (± standard deviation) for capsules with 3%, 4%, and 9% sodium alginate cured under different conditions.  Figure 2 – Disintegration time at pH 7.4: Time to complete capsule opening at pH 7.4 for all samples that resisted pH 6.8.
Figure 2 – Disintegration time at pH 7.4: Time to complete capsule opening at pH 7.4 for all samples that resisted pH 6.8. Figure 3 — SEM analysis of capsule shell cross-sections after curing at 70°C with different relative humidities. A: 70°C – Dry; B: 70°C – 40% RH; C: 70°C – 75% RH
Figure 3 — SEM analysis of capsule shell cross-sections after curing at 70°C with different relative humidities. A: 70°C – Dry; B: 70°C – 40% RH; C: 70°C – 75% RH