Formulation and Delivery - Chemical
(W1030-09-56) Investigating the Impact of Variation in Carbomer Homopolymer Type C (Carbopol® 980) concentration on Diclofenac Sodium Gels’ Microstructure and Skin Permeation

Anuradha Dey, MS (she/her/hers)
Graduate PhD Student
Mercer University
Atlanta, Georgia, United States
Anuradha Dey, MS (she/her/hers)
Graduate PhD Student
Mercer University
Atlanta, Georgia, United States- NV
Nethra Viswaroopan, BS
PhD student
Mercer University
Atlanta, Georgia, United States 
Tanvi Karve, Ph.D. (she/her/hers)
Graduate PhD student
Mercer University
Atlanta, Georgia, United States- NS
Nisha Shrestha, Ph.D. (she/her/hers)
Graduate PhD student
Mercer University
Atlanta, Georgia, United States - MN
Mengmeng Niu, Ph.D.
Senior Pharmacologist
US Food and Drug Administration
Maryland, Maryland, United States 
Priyanka Ghosh, Ph.D.
Lead Pharmacologist at Office of Research and Standards
US Food and Drug Administration
Maryland, Maryland, United States
Ajay K. K. Banga, PhD
Professor
Mercer University
Atlanta, Georgia, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: Gels of diclofenac sodium (0.5% w/w) were prepared using a manufacturing process after optimizing several parameters as depicted in Figure 1. The gels were formulated by varying Q2 of Carbopol® 980, the main gelling agent along with other ingredients as seen in Table 1. Formulation 1 (F1) containing 0.5% w/w of Carbopol® 980 was considered as the hypothetical reference gel. The concentrations of Carbopol® 980 in the rest of formulations represent ± 10% (F3, F4) and ± 25% (F2, F5) quantitative differences compared to 0.5% w/w in the reference gel. Visual appearance, optical microscopy, pH, texture analysis, specific gravity and rheological properties were assessed (n=3) to identify potential differences in Q3 properties between the gels compared to the reference formulation. Following this, a finite dose IVPT study was performed using human cadaver skin (3 donors, 4 replicates per donor) for a duration of 24h after optimizing study parameters.
Results: After optimizing the manufacturing parameters, clear, crystal-free stable gels were prepared for all five formulations (F1-F5) (Figure 2). The pH of the gels was monitored, and the change was found to be minimal over 7 days (Figure 2). Texture analysis including adhesiveness, cohesiveness and hardness of the gels was determined, and the results showed that 1) Increasing Carbopol® 980 concentration decreased the adhesiveness; 2) Values of cohesiveness and hardness were comparable amongst the five formulations. Rheological study results showed that 1) All five formulations depict non-Newtonian behavior; 2) Yield stress and viscosity increased with increasing concentration of Carbopol® 980 in the gels; 3) Gels with higher concentrations of Carbopol® 980 have higher storage modulus; lastly, 4) Gels with higher concentrations of Carbopol® 980 have higher crossover points, suggesting that they have stronger chemical or physical bonding making them more resistant to deformation. The IVPT results demonstrated comparable permeation profiles between F2 and F1, and between F5 and F1.
Conclusion: The tests performed in this study suggest that Q2 changes in Carbopol® 980 influence Q3 attributes of diclofenac sodium topical gels which were observed as differences in texture and rheological profiles. Concentration changes of ± 25% in the gelling agent from the reference (0.5% w/w) influence the Q3 properties of the gels more than the ± 10% change. However, these changes did not result in significant differences in skin permeation of diclofenac sodium from the topical gels.
References: 1. FDA, CDER, In Vitro Release Test Studies for Topical Drug Products Submitted in ANDAs Guidance for Industry DRAFT GUIDANCE. https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs (accessed March 28, 2025).
2. FDA, CDER, In Vitro Permeation Test Studies for Topical Drug Products Submitted in ANDAs Guidance for Industry DRAFT GUIDANCE.
https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs (accessed March 28, 2025).
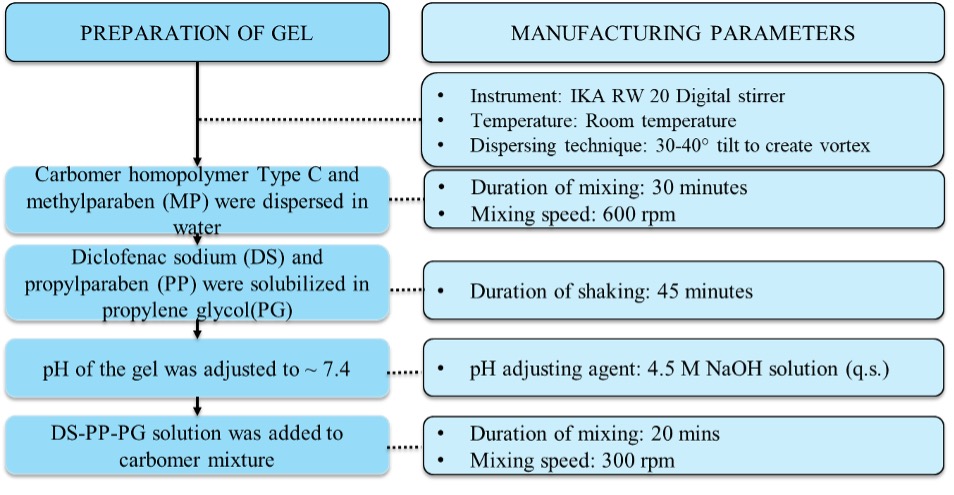 Figure 1. Manufacturing method of diclofenac sodium topical gels containing Carbopol® 980 (F1-F5)
Figure 1. Manufacturing method of diclofenac sodium topical gels containing Carbopol® 980 (F1-F5)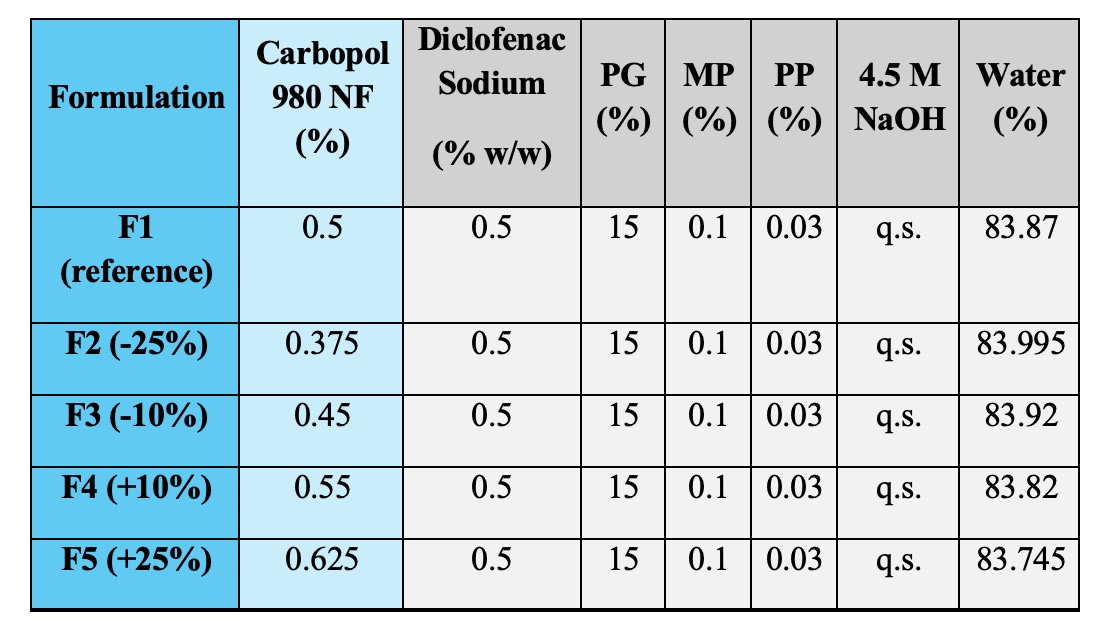 Table 1. Formulation composition of diclofenac sodium topical gels containing Carbopol® 980 (F1-F5)
Table 1. Formulation composition of diclofenac sodium topical gels containing Carbopol® 980 (F1-F5)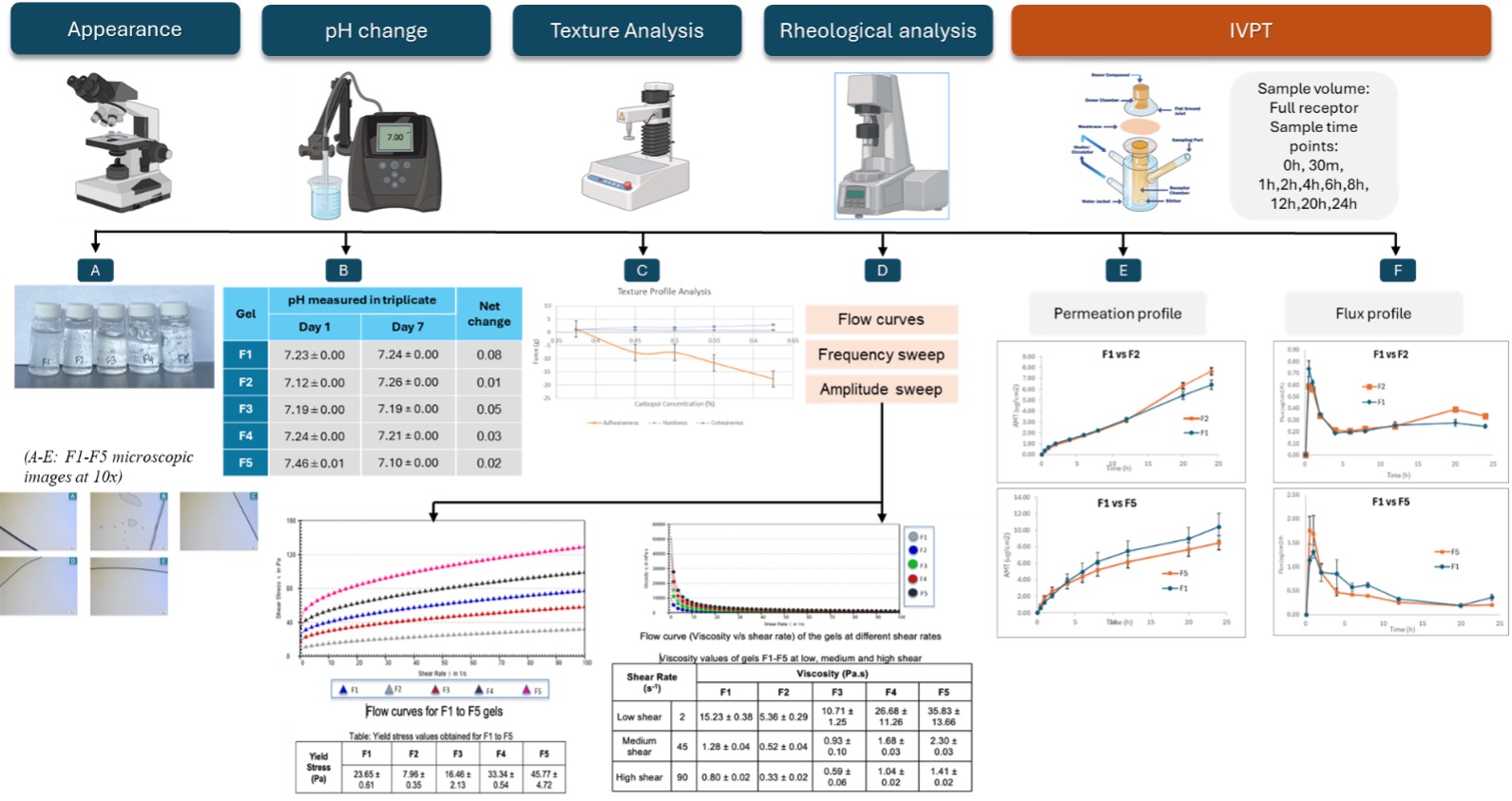 Figure 2: Q3 characterization and IVPT results for diclofenac sodium topical gels (F1-F5). (A): Visual appearance and microscopic images; (B) pH (n=3); (C): texture analysis (Mean ± SD, n=3); (D) rheological analysis: flow curves (shear stress vs/ shear rate, viscosity vs/ shear rate, data represents mean values, n=3) depicting yield stress values (Mean ± SD, n=3) and viscosity values (Mean ± SD, n=3); (E) IVPT drug permeation profile (Mean ± SE, 3 donors, 4 replicates per donor); (F) IVPT flux profile (Mean ± SE, 3 donors, 4 replicates per donor)
Figure 2: Q3 characterization and IVPT results for diclofenac sodium topical gels (F1-F5). (A): Visual appearance and microscopic images; (B) pH (n=3); (C): texture analysis (Mean ± SD, n=3); (D) rheological analysis: flow curves (shear stress vs/ shear rate, viscosity vs/ shear rate, data represents mean values, n=3) depicting yield stress values (Mean ± SD, n=3) and viscosity values (Mean ± SD, n=3); (E) IVPT drug permeation profile (Mean ± SE, 3 donors, 4 replicates per donor); (F) IVPT flux profile (Mean ± SE, 3 donors, 4 replicates per donor)