Manufacturing and Analytical Characterization - Chemical
(W1130-02-13) Multi-Compartment In Vitro Dissolution Testing to Study the Potential Impact of API Changes on Drug-in-Capsule Absorption for Mobocertinib, a Weak Base with Complex pH-Dependent Solubility
- MP
Michael E. Perlman, PhD
Associate Scientific Fellow, Drug Product Development and Devices/Analytical Development
Takeda Pharmaceutical Company Limited
Needham, Massachusetts, United States - MP
Michael E. Perlman, PhD
Associate Scientific Fellow, Drug Product Development and Devices/Analytical Development
Takeda Pharmaceutical Company Limited
Needham, Massachusetts, United States - PG
Prajakta Gadgil
Senior Scientist, Analytical Development
Takeda Pharmaceutical Company Limited
Cambridge, Massachusetts, United States 
Dean P. Phelps, JD MBA (he/him/his)
US Patent Attorney
Takeda Pharmaceuticals & Adler Pollock & Sheehan P.C.
Salem, Massachusetts, United States- MH
Melissa Hollfelder
Analytical Development
Takeda Pharmaceutical Company Limited
Waltham, Massachusetts, United States - AM
Andy Moy
Analytical Development
Takeda Pharmaceutical Company Limited
Boston, Massachusetts, United States - LD
Landon Durak
Senior Scientist, Synthetic Molecule Process Development
Takeda Pharmaceutical Company Limited
New Haven, Connecticut, United States - JQ
Justin Quon
Senior Staff Engineer, Synthetic Molecule Process Development
Takeda Pharmaceutical Company Limited
New Haven, Connecticut, United States - YK
Yuki Kodono
Japan Regional Product Lifecycle Management
Takeda Pharmaceutical Company Limited
Yodogawa-ku, Osaka, Japan - IA
Ian Armitage
Head, AD Early Development US
Takeda Pharmaceutical Company Limited
Cambridge, Massachusetts, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: Equilibrium solubilities were obtained in 100 mM sodium phosphate buffer or biorelevant fluids, with shorter experiment times required at higher pH values due to rapid degradation. Biorelevant dissolution was performed using the apparatus shown in Figure 1. The gastric compartment contained 250 mL Milli-Q® water and 50 mL of pH 2 or 4.5 FaSSGF at 37°C, stirred with a paddle at 80 rpm. Fluid was transferred by a peristaltic pump at the rate of human gastric emptying (15 min half-life) to the duodenal compartment, which contained 50 mL of pH 6.5 or 7.5 FaSSIF. Transfer was stopped at 75 min. The volume in the duodenal compartment was kept constant by continuous transfer to the jejunal compartment (containing 100 mL water). The pH and bile concentration in the duodenal compartment were controlled by secretion of 4x concentrated FaSSIF. The dissolved concentration in each compartment was monitored by UV absorption using a µDiss™ probe (Pion, Inc.) and/or by HPLC analysis.
Results: Equilibrium solubilities at 37°C (Table 1) decreased with increasing pH, but increased again at pH 6.4 -6.8, followed by a sharp decrease. There was also reversible dimerization (based on LC-MS) observed at pH 6.5 that increased with concentration. A 160 mg dose (clinical dose) of each DiC was added to the gastric compartment (Figure 1) containing pH 4.5 FaSSGF/water (pH ~5 with water), and then transferred to the duodenal compartment (pH 6.5 FaSSIF). The concentrations in all three compartments (Figure 2) were very similar for Process A and C DiC (confirmed using triplicate experiments), but Process B DiC exhibited 10-15 min slower gastric dissolution (HPLC) and 20% lower duodenal levels (UV). Superimposable duodenal curves were observed for Process A, B and C DiC when the 3-compartment experiment was performed with pH 2.0 FaSSGF/water (pH ~2.5 with water) in the gastric compartment. When pH 7.5 FaSSIF was in the duodenal compartment (for API alone), precipitation occurred when the duodenal pH reached 7.0.
Conclusion: As a result of its high solubility at pH 1-6.8 (and high in vitro permeability) mobocertinib is considered a BCS I compound. There was an unexpected peak in solubility at ~ pH 6.5 that appears to promote dimerization. For in vitro transfer studies, similar results were observed for transfer from gastric pH 4.5 to duodenal pH 6.5 and suggest that Process A and C capsules should perform similarly in cancer patients, which often exhibit elevated gastric pH (e.g. due to use of proton pump inhibitors).3 Slower dissolution for Process B capsules in this apparatus is presumably due to the >2 fold higher API mean particle size (80 µm) relative to Process A and C API (~30 µm), with similar trends for d90. However, considering the long human plasma Tmax (4-6 hr) for mobocertinib,4 changes of this magnitude may not have an impact on clinical PK. Slower absorption does suggest that luminal concentrations will not be greatly overestimated by lack of an absorptive process in this apparatus, which is a common concern for in vitro transfer systems.1 Results for transfer from gastric pH 2.5 to duodenal pH 6.5 indicate that all three process capsules should perform similarly across this pH range in normal healthy subjects. This is consistent with bioequivalence between Process A and B capsules observed in healthy subjects in an rBA study.4,5
References: 1. K. Matsui, Y. Tsume, G. E. Amidon, G.L. Amidon, The Evaluation of In Vitro Drug Dissolution of Commercially Available Oral Dosage Forms for Itraconazole in Gastrointestinal Simulator with Biorelevant Media. J Pharm Sci 105 (2016): 2804-2814.
2. S. Carino, D. Sperry, M. Hawley. Relative bioavailability of three different solid forms of PNU-141659 as determined with the artificial stomach-duodenum
model. J Pharm Sci 99 (2010): 3923– 3930.
3. N. Budha, A. Frymoyer, S. Smelick, J. Jin, M. Yago, M. Dresser, S. Holden, L. Benet, J. Ware. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther 92 (2012): 203-213.
4. S. Zhang, S. Jin, C. Griffin, Z. Feng, J. Lin, M. Baratta, R. Brake, K. Venkatakrishnan, N. Gupta. Single-dose pharmacokinetics and tolerability of the oral epidermal growth factor receptor inhibitor mobocertinib (TAK-788) in healthy volunteers: low-fat meal effect and relative bioavailability of 2 capsule products. Clin Pharmacol Drug Dev 10 (2021):1028-1043.
5. N. Gupta N, P. Pierillas, M. Hanley, S. Zhang, P. Diderichsen. Population pharmacokinetics of mobocertinib in healthy volunteers and patients with
non-small cell lung cancer. CPT Pharmacometrics Syst Pharmacol 11(2022):731-744.
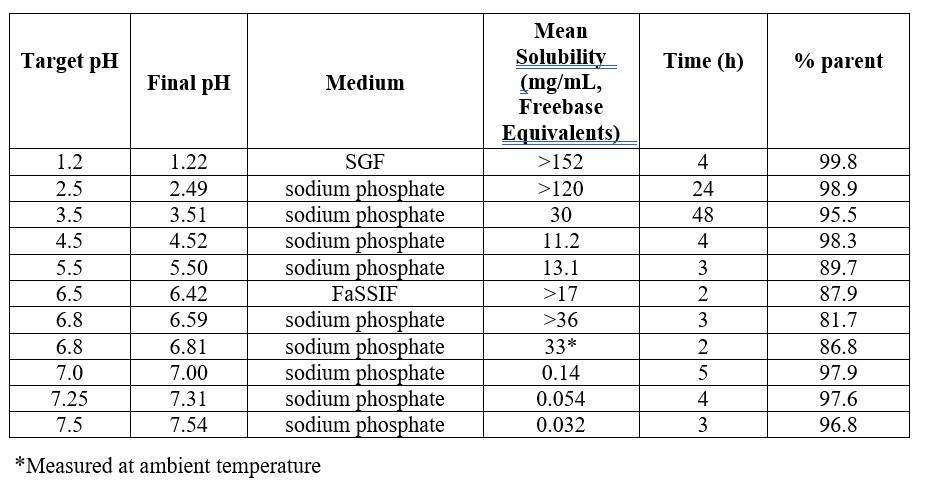 Table 1. pH-solubility data for mobocertinib at 37°C
Table 1. pH-solubility data for mobocertinib at 37°C 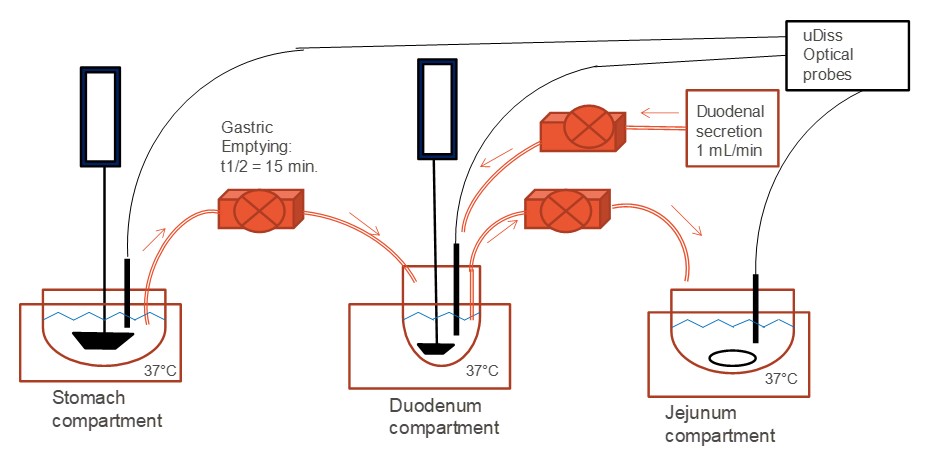 Figure 1. 3-compartment in-vitro dissolution apparatus
Figure 1. 3-compartment in-vitro dissolution apparatus 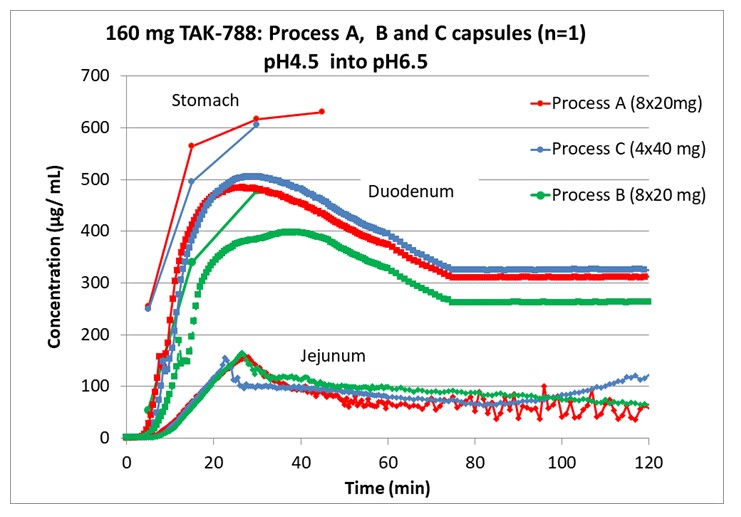 Figure 2. In vitro dissolution of DiC’s in pH 4.5 FaSSGF/water followed by transfer into pH 6.5 FaSSIF
Figure 2. In vitro dissolution of DiC’s in pH 4.5 FaSSGF/water followed by transfer into pH 6.5 FaSSIF 