Discovery and Basic Research
(T1030-03-16) Enhancing Transdermal Delivery of Salbutamol via Optimized Nanoemulsion Formulations: The Impact of Surfactant Selection and Component Ratios
Tuesday, November 11, 2025
10:30 AM - 11:30 AM CT
- OE
Ozge Esen Yigit, MS
PhD Candidate
University of Bonn
Bonn, Nordrhein-Westfalen, Germany 
Alf Lamprecht, PhD
PI
University of Bonn
Bonn, Nordrhein-Westfalen, Germany
Presenting Author(s)
Main Author(s)
Purpose: Transdermal drug delivery systems (TDDs) have traditionally been limited to lipophilic drugs due to the skin’s lipophilic barrier, making hydrophilic drug delivery particularly challenging. However, emerging approaches such as nanoemulsion formulations offer promising strategies to overcome these limitations [1], [2]. This study focused on enhancing the transdermal delivery of salbutamol, a hydrophilic β2-adrenergic agonist widely used for the treatment of asthma and other respiratory conditions but limited by low oral bioavailability and risks of misuse in inhalation therapy. In this study, we aimed to optimize formulation parameters to enhance permeation and provide a patient-friendly alternative administration way for salbutamol.
Methods: Nanoemulsions were prepared using water, Miglyol 812 (a medium-chain triglyceride, MCT) as the oil phase, and either Kolliphor EL or HS 15 as surfactants. Ternary diagrams were designed with 36 data points for each surfactant, varying component proportions to investigate their effects on permeation behavior. Nanoemulsions were obtained via a modified phase inversion temperature (PIT) method, as reported by Lamprecht et al. [3], by heating the mixtures above their phase inversion temperature, followed by gradual cooling to induce spontaneous emulsification. Droplet size and polydispersity index were measured using photon correlation spectroscopy (Horiba Scientific nanoPartica SZ-100), and viscosity was assessed with a modular rheometer (Anton Paar, MCR92). Skin permeation studies were performed using Franz diffusion cells (SES Analysensysteme, Bechenheim, Germany) with excised pig skin, and salbutamol flux was quantified via HPLC. Ternary diagrams were constructed using OriginLab Pro to visualize formulation effects on droplet size and permeation behavior. Data were analyzed using isometric log-ratio (ilr) transformation and generalized additive models (GAM) to evaluate non-linear relationships between formulation composition and permeation outcomes.
Results: Nanoemulsion formulations were successfully prepared using the phase inversion method with varying ratios of water, Miglyol 812, and surfactants (Kolliphor EL or HS 15). Among the Kolliphor EL emulsions (KEs), 17 out of 28 stable formulations exhibited droplet sizes under 100 nm, with all but one remaining below 500 nm. Viscosities of these formulations ranged widely from 3 mPa·s to 9.5 Pa·s, yielding systems from fluid to gel-like consistency. In terms of skin permeation, flux values for KEs varied between 89 and 381 µg/cm²·h, with the highest flux observed at 70% water, 10% Kolliphor EL, and 20% Miglyol. Cumulative drug permeation ranged from 1951 to 5010 µg/cm². HS 15 emulsions (HSEs) showed similar droplet size and stability metrics but generally produced higher permeation rates, with flux values between 177 and 391 µg/cm²·h and cumulative permeation ranging from 3194 to 5254 µg/cm². The highest flux within HSEs was achieved with the same water-dominant ratio as KEs, reinforcing the critical role of water content in enhancing flux. Statistical analysis using Generalized Additive Models (GAM) confirmed significant non-linear relationships between component ratios and flux. Partial dependence plots highlighted water content as the primary factor positively influencing flux in both systems. While Kolliphor EL showed a more predictable relationship with flux, HS 15 induced complex interaction patterns. Miglyol 812 contributed a stabilizing, modest effect. Neither droplet size nor viscosity alone correlated directly with flux, although weak interaction effects between these factors were noted.
Conclusion: This study successfully demonstrates the feasibility of using low-energy PIT methods to develop nanoemulsions capable of significantly enhancing transdermal delivery of hydrophilic drugs. By systematically evaluating surfactant selection and component ratios, these findings suggest that careful modulation of nanoemulsion components can tailor drug release profiles. Our GAM-based modeling provides a predictive framework for formulating stable and efficient transdermal systems. These insights not only facilitate future formulation strategies for salbutamol but also offer valuable guidance for optimizing future transdermal formulations and advancing hydrophilic drug delivery strategies.
References: [1] Marques, L. and Vale, N. (2022) ‘Salbutamol in the management of asthma: A Review’, International Journal of Molecular Sciences, 23(22), p. 14207. doi:10.3390/ijms232214207.
[2] Shaker, D.S.; Ishak, R.A.H.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A Review on Mechanisms for the Transdermal Delivery of Hydrophobic and Hydrophilic Drugs. Sci. Pharm. 2019, 87, 17. https://doi.org/10.3390/scipharm87030017
[3] Lamprecht, A., Bouligand, Y. and Benoit, J.-P. (2002) ‘New lipid nanocapsules exhibit sustained release properties for Amiodarone’, Journal of Controlled Release, 84(1–2), pp. 59–68. doi:10.1016/s0168-3659(02)00258-4.
Acknowledgements: This work was supported by a scholarship from the Ministry of National Education of the Republic of Türkiye.
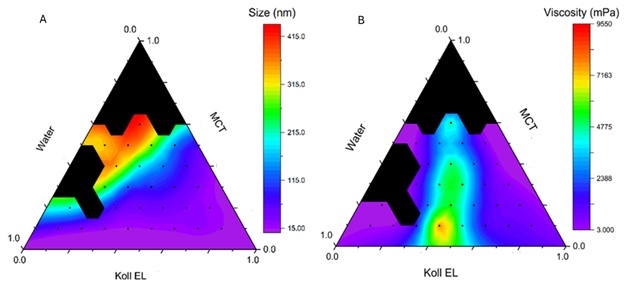
Figure 1. Ternary diagrams of mean droplet size (A) and viscosity (B) of KEs. The black regions indicate data points representing phase-separated formulations.
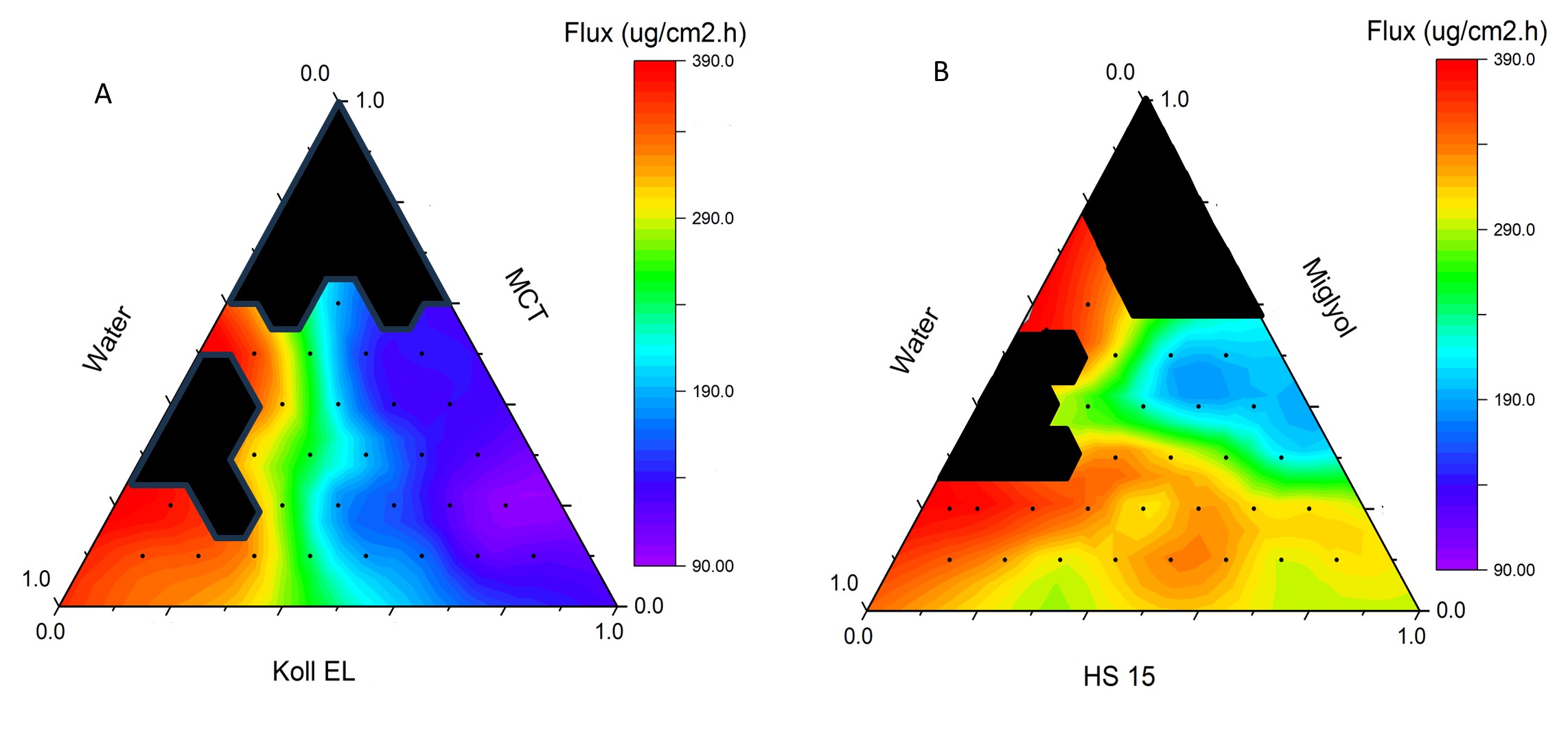
Figure 2. Ternary diagrams of permeation profiles of KEs (A) and HSEs (B). Each data point shows the average flux of triplicated permeation results. The black regions indicate data points representing phase-separated formulations.
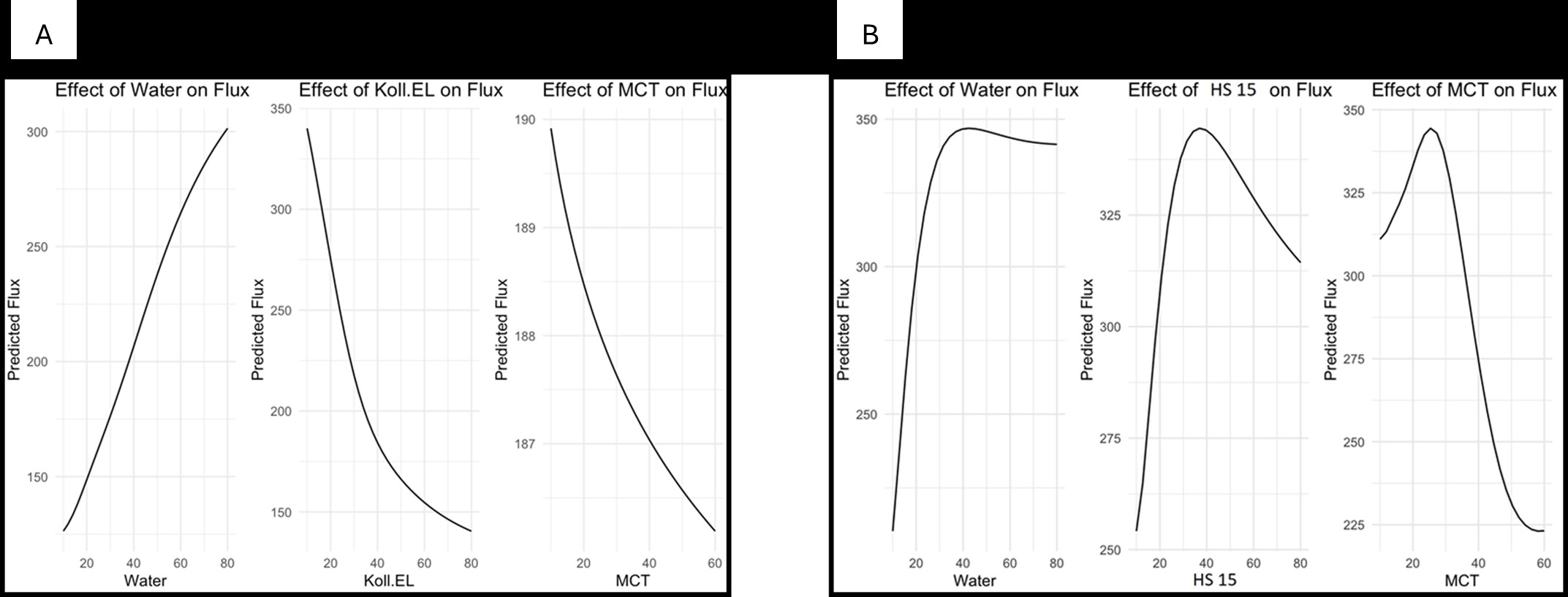
Figure 3. Partial dependence plots for water, MCT, and surfactants, Kolliphor EL (A) and HS 15 (B), on flux
Methods: Nanoemulsions were prepared using water, Miglyol 812 (a medium-chain triglyceride, MCT) as the oil phase, and either Kolliphor EL or HS 15 as surfactants. Ternary diagrams were designed with 36 data points for each surfactant, varying component proportions to investigate their effects on permeation behavior. Nanoemulsions were obtained via a modified phase inversion temperature (PIT) method, as reported by Lamprecht et al. [3], by heating the mixtures above their phase inversion temperature, followed by gradual cooling to induce spontaneous emulsification. Droplet size and polydispersity index were measured using photon correlation spectroscopy (Horiba Scientific nanoPartica SZ-100), and viscosity was assessed with a modular rheometer (Anton Paar, MCR92). Skin permeation studies were performed using Franz diffusion cells (SES Analysensysteme, Bechenheim, Germany) with excised pig skin, and salbutamol flux was quantified via HPLC. Ternary diagrams were constructed using OriginLab Pro to visualize formulation effects on droplet size and permeation behavior. Data were analyzed using isometric log-ratio (ilr) transformation and generalized additive models (GAM) to evaluate non-linear relationships between formulation composition and permeation outcomes.
Results: Nanoemulsion formulations were successfully prepared using the phase inversion method with varying ratios of water, Miglyol 812, and surfactants (Kolliphor EL or HS 15). Among the Kolliphor EL emulsions (KEs), 17 out of 28 stable formulations exhibited droplet sizes under 100 nm, with all but one remaining below 500 nm. Viscosities of these formulations ranged widely from 3 mPa·s to 9.5 Pa·s, yielding systems from fluid to gel-like consistency. In terms of skin permeation, flux values for KEs varied between 89 and 381 µg/cm²·h, with the highest flux observed at 70% water, 10% Kolliphor EL, and 20% Miglyol. Cumulative drug permeation ranged from 1951 to 5010 µg/cm². HS 15 emulsions (HSEs) showed similar droplet size and stability metrics but generally produced higher permeation rates, with flux values between 177 and 391 µg/cm²·h and cumulative permeation ranging from 3194 to 5254 µg/cm². The highest flux within HSEs was achieved with the same water-dominant ratio as KEs, reinforcing the critical role of water content in enhancing flux. Statistical analysis using Generalized Additive Models (GAM) confirmed significant non-linear relationships between component ratios and flux. Partial dependence plots highlighted water content as the primary factor positively influencing flux in both systems. While Kolliphor EL showed a more predictable relationship with flux, HS 15 induced complex interaction patterns. Miglyol 812 contributed a stabilizing, modest effect. Neither droplet size nor viscosity alone correlated directly with flux, although weak interaction effects between these factors were noted.
Conclusion: This study successfully demonstrates the feasibility of using low-energy PIT methods to develop nanoemulsions capable of significantly enhancing transdermal delivery of hydrophilic drugs. By systematically evaluating surfactant selection and component ratios, these findings suggest that careful modulation of nanoemulsion components can tailor drug release profiles. Our GAM-based modeling provides a predictive framework for formulating stable and efficient transdermal systems. These insights not only facilitate future formulation strategies for salbutamol but also offer valuable guidance for optimizing future transdermal formulations and advancing hydrophilic drug delivery strategies.
References: [1] Marques, L. and Vale, N. (2022) ‘Salbutamol in the management of asthma: A Review’, International Journal of Molecular Sciences, 23(22), p. 14207. doi:10.3390/ijms232214207.
[2] Shaker, D.S.; Ishak, R.A.H.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A Review on Mechanisms for the Transdermal Delivery of Hydrophobic and Hydrophilic Drugs. Sci. Pharm. 2019, 87, 17. https://doi.org/10.3390/scipharm87030017
[3] Lamprecht, A., Bouligand, Y. and Benoit, J.-P. (2002) ‘New lipid nanocapsules exhibit sustained release properties for Amiodarone’, Journal of Controlled Release, 84(1–2), pp. 59–68. doi:10.1016/s0168-3659(02)00258-4.
Acknowledgements: This work was supported by a scholarship from the Ministry of National Education of the Republic of Türkiye.

Figure 1. Ternary diagrams of mean droplet size (A) and viscosity (B) of KEs. The black regions indicate data points representing phase-separated formulations.

Figure 2. Ternary diagrams of permeation profiles of KEs (A) and HSEs (B). Each data point shows the average flux of triplicated permeation results. The black regions indicate data points representing phase-separated formulations.

Figure 3. Partial dependence plots for water, MCT, and surfactants, Kolliphor EL (A) and HS 15 (B), on flux
