Formulation and Delivery - Chemical
(M0930-08-54) Development of a Patient Friendly, Pediatric Formulation of Xalkori Leveraging a Multiparticulate Formulation Approach and Quality By Design Principles
Monday, November 10, 2025
9:30 AM - 10:30 AM CT
- HS
Hannah Sullivan, BS
Principal Investigator, Manager CMC
Lonza
Bend, Oregon, United States - HS
Hannah Sullivan, BS
Principal Investigator, Manager CMC
Lonza
Bend, Oregon, United States - NC
Natalie Culver, MS
Senior Scientist / Drug Product Design – Formulation and Process Design
Pfizer, Inc.
Groton, Connecticut, United States 
Xiang Zhang, PhD
Sr Manager Biostatistics
Pfizer, Inc.
Groton, Connecticut, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: A pediatric dosage form for crizotinib (Xalkori) was developed and commercialized using quality by design principles in a material sparing fashion [1]. Statistical design of experiments was leveraged to optimize the manufacturing process. The dosage form consists of high drug loaded spherical multiparticulates (microspheres or pellets) that are coated and encapsulated in capsules for opening for dosing flexibility. This multiparticulate formulation addresses key palatability difficulties encountered by an oral solution formulation [2, 3], and enhances the pediatric patient experience with this life saving medicine.
Methods: Design of experiments (DoE) and data analysis was performed in Design Expert (Stat-Ease®) and JMP® (JMP® Statistical Discovery). Crizotinib coated microspheres are manufactured in a multi-step process: 1) blending of the raw materials; 2) melt extrusion, forming a liquid suspension (melt) with the API; 3) spray congealing of the melt to form uncoated microspheres; 4) screening to select the desired particle size; 5) film coating of the microspheres. The coated microspheres are then encapsulated in capsules for opening.
Results: The melt-spray-congeal, fluid bed and encapsulation processes were studied using statistical design of experiments. MSC: The melt-spray-congeal (MSC) manufacturing process was explored through a fractional factorial DoE [1]. Additional confirmation of the process space and bridging of the process to the commercial equipment used a full factorial DoE on cooling air flow rate and air temperature, with targeted run conditions at the process parameter action limits. This demonstrated that the process produced acceptable material when operated to manufacture small and large particle size distributions. The particle size results show consistent distributions with a tight span across the different run conditions, which reflects the robustness of the process. Fluid bed: Development batch history for the fluid bed coating process identified a process corner failure with respect to high agglomeration (material retained as twins or triplets on a certain mesh screen). In response, a single fluid bed batch experiment using a fractional factorial DoE (25-2 plus two center points) was performed to reduce the process space slightly and to nominate process limits. Agglomeration and potency were used as response variables. Spray rate and product temperature were found to be statistically significant for agglomeration but these parameters did not influence potency. Encapsulation: An I-optimal DoE was used to evaluate all of the effects and some of the two-way interactions for the six factors that control the volumetric dosator encapsulation process. Material usage was minimized by running the encapsulator for only enough time to reach steady state and to take a sample of 300 capsules for analysis. Filled capsule relative standard deviation (RSD) was used as the response variable to evaluate the success of the experiment. Most run conditions resulted in a RSD of less than the proposed limit of 2.25%. Of note were two repeats of the same run conditions at the lowest fill vacuum (-225 mbar) and highest air knife pressure (1200 mbar), exceeding the RSD limit with results of 3.51% and 5.26% RSD. The DoE model was used to select the upper limit of the air knife parameter, which was most statistically significant (p < 0.001), as not more than 1180 mbar, to avoid the worst-case corner of the process space at an air knife pressure of 1200 mbar. In order to confirm the validity of using filled capsule weight RSD as a surrogate for content uniformity by HPLC, USP < 905 > methodology was used to evaluate the content uniformity of the centerline and highest RSD condition. Both the centerline and highest RSD conditions (lowest fill vacuum and highest air knife pressure) passed the USP < 905 > content uniformity acceptance value upper limit of 15. A process demonstration batch was manufactured by operating each third of the batch at different processing conditions. The first third of the batch was operated at target conditions (condition 1). The middle third of the batch was operated at a process space edge (condition 2) where infeed level, fill vacuum and air knife pressure were set at the process control limits on one side of the process space. The last third of the batch was operated at a process space edge (condition 3) where infeed level, fill vacuum and air knife pressure were set at the process control limits on the opposite side from condition 2. This batch demonstrated that the operating space edges produced acceptable product, passing RSD criteria and with good content uniformity as tested by ASTM E2810 methodology.
Conclusion: A limited number of development batches produced using commercial scale equipment were leveraged to design, understand, and verify the manufacturing process space, which resulted in a pediatric product with exceptional content uniformity (a 95% confidence and 99% probability of passing USP < 905 > content uniformity testing for future batches).
References: [1] Jeremy Bartlett, Natalie Culver, Xiang Zhang, Brett Waybrant, Hannah Sullivan and Logan Howell. Commercialization of the Xalkori Pediatric Multiparticulate Procut Using Quality By Design Principles Pharamceutics, 2024, 16(8), 1027.
[2] Greengard, E.; Mosse, Y.P.; Liu, X.; Minard, C.G.; Reid, J.M.; Voss, S.; Wilner, K.; Fox, E.; Balis, F.; Blaney, S.M., et al. Safety, tolerability and pharmacokinetics of crizotinib in combination with cytotoxic chemotherapy for pediatric patients with refractory solid tumors or anaplastic large cell lymphoma (alcl): A children's oncology group phase 1 consortium study (advl1212). Cancer chemotherapy and pharmacology 2020, 86, 829-840.10.1007/s00280-020-04171-4
[3] Greengard Emily Gustava, M.Y.P., Liu Xiaowei, et al. Safety and tolerability of crizotinib in combination with chemotherapy for relapsed or refractory solid tumors or anaplastic large cell lymphoma: A children’s oncology group phase i consortium study. Journal of Clinical Oncology 2015, 33.
Acknowledgements: We would like to thank the Pfizer R&D and Pfizer Global Manufacturing colleagues in Groton USA and across Pfizer’s global network whose work has supported the development of this manufacturing process in general, and a special thanks to those who have kept a focus on the pediatric patient for the development of the crizotinib pediatric dosage form specifically. We would also like to thank the development and manufacturing colleagues at Lonza, especially the Lonza Bend Oregon site, whose work has supported the process development presented here.
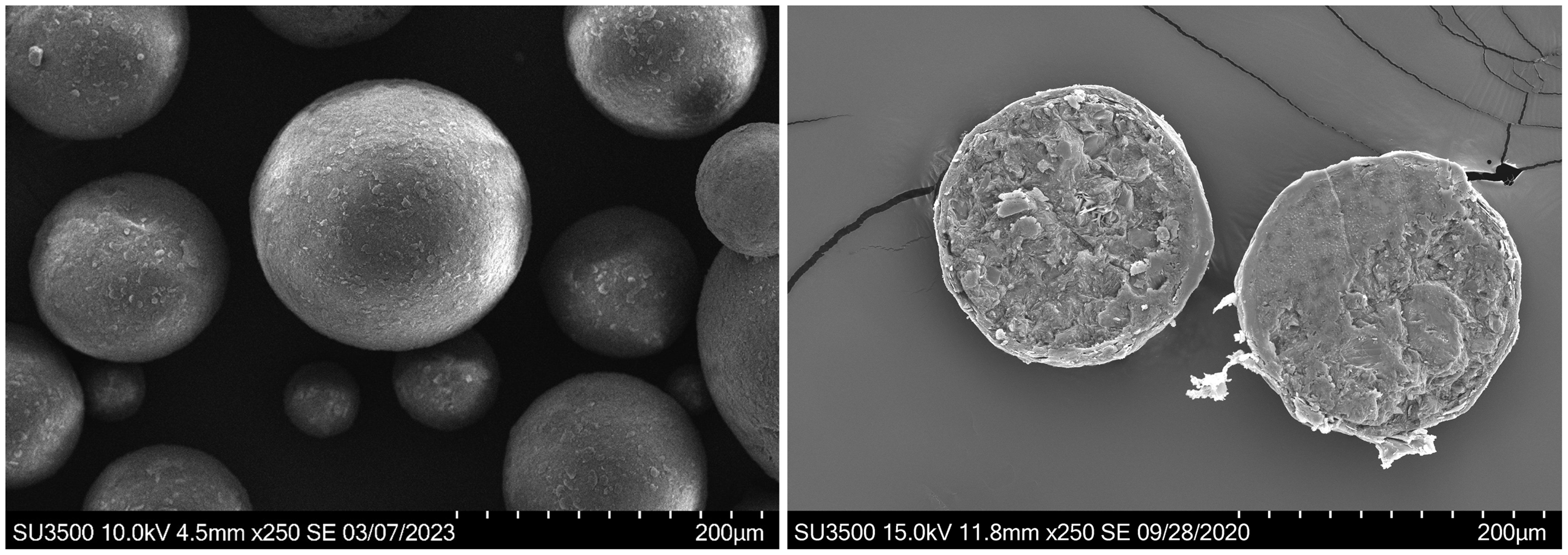 Left: Uncoated microspheres produced during melt-spray-congeal. Right: Cross sectioned coated microsphere after fluid bed coating, showing coating thickness.
Left: Uncoated microspheres produced during melt-spray-congeal. Right: Cross sectioned coated microsphere after fluid bed coating, showing coating thickness.
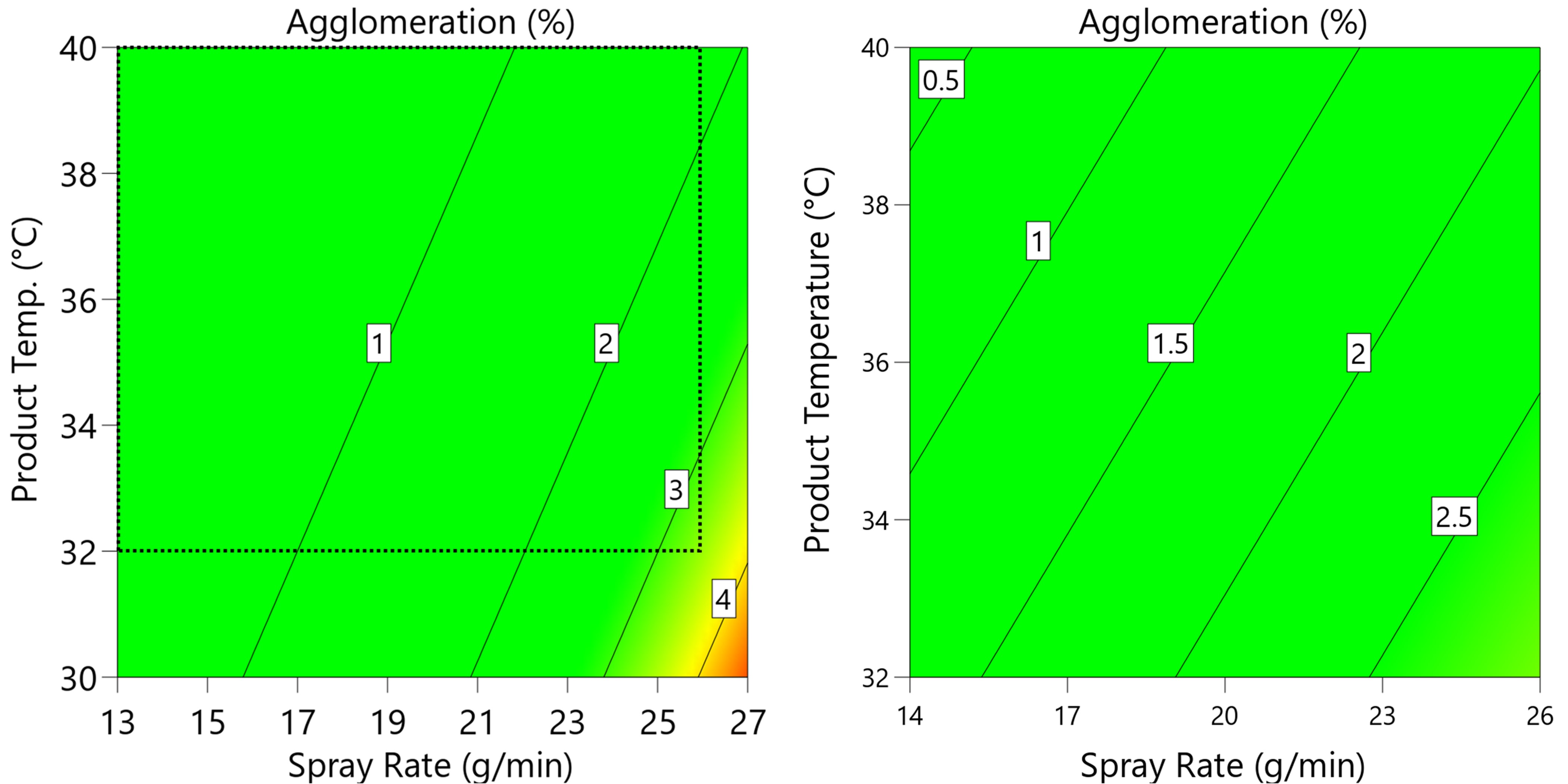 Left: Development batch experience, process data summarized from 3 batches, showing edge of failure with respect to agglomeration. Right: Fractional factorial DoE reducing the process space to avoid the edge of failure. Dashed lines are nominated process limits.
Left: Development batch experience, process data summarized from 3 batches, showing edge of failure with respect to agglomeration. Right: Fractional factorial DoE reducing the process space to avoid the edge of failure. Dashed lines are nominated process limits.
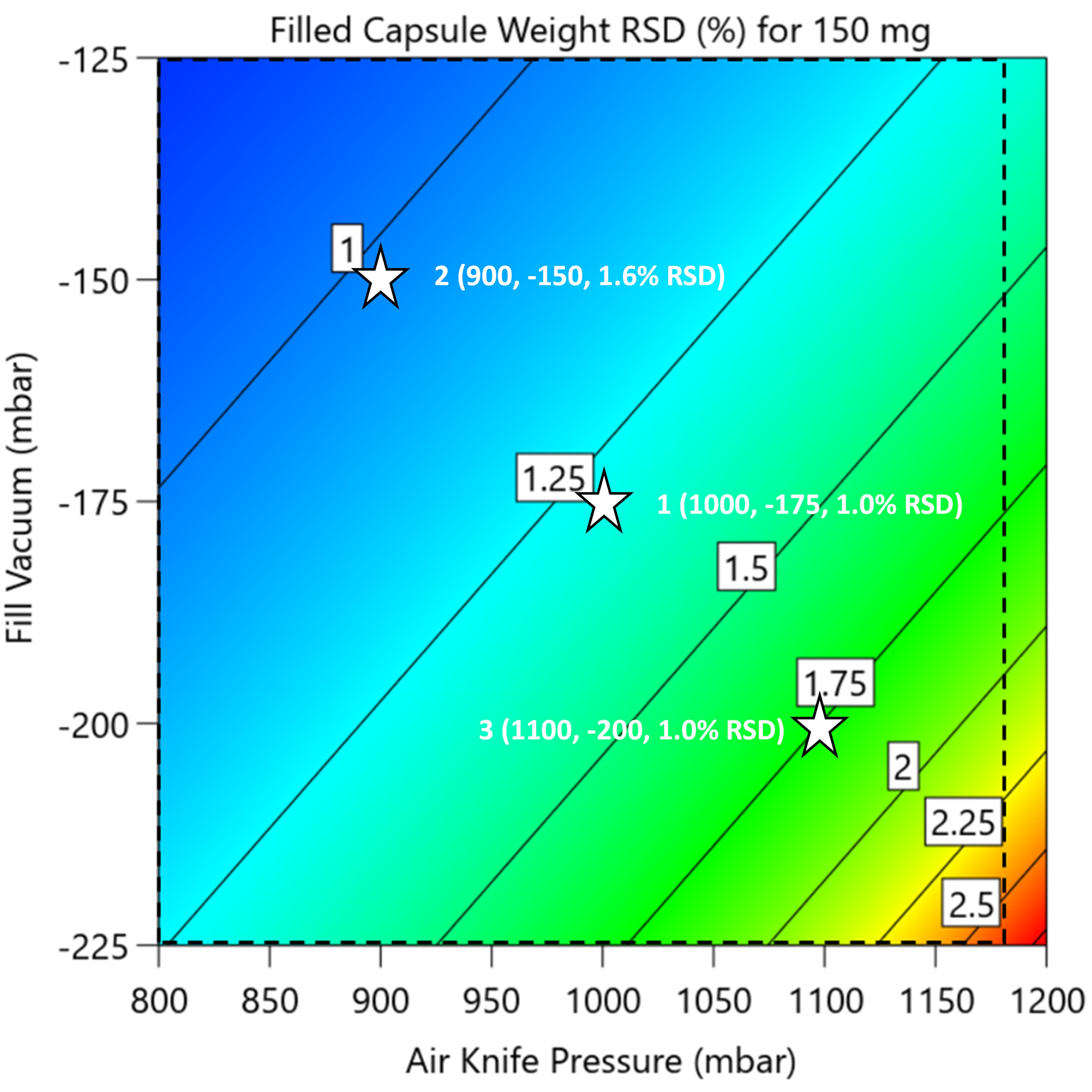 Process map for encapsulation, showing filled capsule weight RSD against the two critical process parameters, air knife pressure and fill vacuum, with infeed level set to 11.5 mm (worst case). Stars denote data from the process demonstration batch. Dashed lines are nominated process limits.
Process map for encapsulation, showing filled capsule weight RSD against the two critical process parameters, air knife pressure and fill vacuum, with infeed level set to 11.5 mm (worst case). Stars denote data from the process demonstration batch. Dashed lines are nominated process limits.
Methods: Design of experiments (DoE) and data analysis was performed in Design Expert (Stat-Ease®) and JMP® (JMP® Statistical Discovery). Crizotinib coated microspheres are manufactured in a multi-step process: 1) blending of the raw materials; 2) melt extrusion, forming a liquid suspension (melt) with the API; 3) spray congealing of the melt to form uncoated microspheres; 4) screening to select the desired particle size; 5) film coating of the microspheres. The coated microspheres are then encapsulated in capsules for opening.
Results: The melt-spray-congeal, fluid bed and encapsulation processes were studied using statistical design of experiments. MSC: The melt-spray-congeal (MSC) manufacturing process was explored through a fractional factorial DoE [1]. Additional confirmation of the process space and bridging of the process to the commercial equipment used a full factorial DoE on cooling air flow rate and air temperature, with targeted run conditions at the process parameter action limits. This demonstrated that the process produced acceptable material when operated to manufacture small and large particle size distributions. The particle size results show consistent distributions with a tight span across the different run conditions, which reflects the robustness of the process. Fluid bed: Development batch history for the fluid bed coating process identified a process corner failure with respect to high agglomeration (material retained as twins or triplets on a certain mesh screen). In response, a single fluid bed batch experiment using a fractional factorial DoE (25-2 plus two center points) was performed to reduce the process space slightly and to nominate process limits. Agglomeration and potency were used as response variables. Spray rate and product temperature were found to be statistically significant for agglomeration but these parameters did not influence potency. Encapsulation: An I-optimal DoE was used to evaluate all of the effects and some of the two-way interactions for the six factors that control the volumetric dosator encapsulation process. Material usage was minimized by running the encapsulator for only enough time to reach steady state and to take a sample of 300 capsules for analysis. Filled capsule relative standard deviation (RSD) was used as the response variable to evaluate the success of the experiment. Most run conditions resulted in a RSD of less than the proposed limit of 2.25%. Of note were two repeats of the same run conditions at the lowest fill vacuum (-225 mbar) and highest air knife pressure (1200 mbar), exceeding the RSD limit with results of 3.51% and 5.26% RSD. The DoE model was used to select the upper limit of the air knife parameter, which was most statistically significant (p < 0.001), as not more than 1180 mbar, to avoid the worst-case corner of the process space at an air knife pressure of 1200 mbar. In order to confirm the validity of using filled capsule weight RSD as a surrogate for content uniformity by HPLC, USP < 905 > methodology was used to evaluate the content uniformity of the centerline and highest RSD condition. Both the centerline and highest RSD conditions (lowest fill vacuum and highest air knife pressure) passed the USP < 905 > content uniformity acceptance value upper limit of 15. A process demonstration batch was manufactured by operating each third of the batch at different processing conditions. The first third of the batch was operated at target conditions (condition 1). The middle third of the batch was operated at a process space edge (condition 2) where infeed level, fill vacuum and air knife pressure were set at the process control limits on one side of the process space. The last third of the batch was operated at a process space edge (condition 3) where infeed level, fill vacuum and air knife pressure were set at the process control limits on the opposite side from condition 2. This batch demonstrated that the operating space edges produced acceptable product, passing RSD criteria and with good content uniformity as tested by ASTM E2810 methodology.
Conclusion: A limited number of development batches produced using commercial scale equipment were leveraged to design, understand, and verify the manufacturing process space, which resulted in a pediatric product with exceptional content uniformity (a 95% confidence and 99% probability of passing USP < 905 > content uniformity testing for future batches).
References: [1] Jeremy Bartlett, Natalie Culver, Xiang Zhang, Brett Waybrant, Hannah Sullivan and Logan Howell. Commercialization of the Xalkori Pediatric Multiparticulate Procut Using Quality By Design Principles Pharamceutics, 2024, 16(8), 1027.
[2] Greengard, E.; Mosse, Y.P.; Liu, X.; Minard, C.G.; Reid, J.M.; Voss, S.; Wilner, K.; Fox, E.; Balis, F.; Blaney, S.M., et al. Safety, tolerability and pharmacokinetics of crizotinib in combination with cytotoxic chemotherapy for pediatric patients with refractory solid tumors or anaplastic large cell lymphoma (alcl): A children's oncology group phase 1 consortium study (advl1212). Cancer chemotherapy and pharmacology 2020, 86, 829-840.10.1007/s00280-020-04171-4
[3] Greengard Emily Gustava, M.Y.P., Liu Xiaowei, et al. Safety and tolerability of crizotinib in combination with chemotherapy for relapsed or refractory solid tumors or anaplastic large cell lymphoma: A children’s oncology group phase i consortium study. Journal of Clinical Oncology 2015, 33.
Acknowledgements: We would like to thank the Pfizer R&D and Pfizer Global Manufacturing colleagues in Groton USA and across Pfizer’s global network whose work has supported the development of this manufacturing process in general, and a special thanks to those who have kept a focus on the pediatric patient for the development of the crizotinib pediatric dosage form specifically. We would also like to thank the development and manufacturing colleagues at Lonza, especially the Lonza Bend Oregon site, whose work has supported the process development presented here.
 Left: Uncoated microspheres produced during melt-spray-congeal. Right: Cross sectioned coated microsphere after fluid bed coating, showing coating thickness.
Left: Uncoated microspheres produced during melt-spray-congeal. Right: Cross sectioned coated microsphere after fluid bed coating, showing coating thickness.  Left: Development batch experience, process data summarized from 3 batches, showing edge of failure with respect to agglomeration. Right: Fractional factorial DoE reducing the process space to avoid the edge of failure. Dashed lines are nominated process limits.
Left: Development batch experience, process data summarized from 3 batches, showing edge of failure with respect to agglomeration. Right: Fractional factorial DoE reducing the process space to avoid the edge of failure. Dashed lines are nominated process limits.  Process map for encapsulation, showing filled capsule weight RSD against the two critical process parameters, air knife pressure and fill vacuum, with infeed level set to 11.5 mm (worst case). Stars denote data from the process demonstration batch. Dashed lines are nominated process limits.
Process map for encapsulation, showing filled capsule weight RSD against the two critical process parameters, air knife pressure and fill vacuum, with infeed level set to 11.5 mm (worst case). Stars denote data from the process demonstration batch. Dashed lines are nominated process limits. 