Formulation and Delivery - Biomolecular
(M1030-08-49) Polymers as the Stabilizing Excipient for Spray-Dried Solid-State Protein Formulations
- CP
Chanakya D Patil, MS
Graduate Student
Purdue University
West Lafayette, Indiana, United States - CP
Chanakya D Patil, MS
Graduate Student
Purdue University
West Lafayette, Indiana, United States - YH
Yijing Huang, MS
Graduate Student
Purdue University
West Lafayette, Indiana, United States 
Kinnari Santosh Arte, PhD (she/her/hers)
Scientist I
Alexion - AstraZeneca Rare Disease
Hamden, Connecticut, United States- HR
Harshil K Renawala, Ph.D.
Associate Principal Scientist
Merck & Co., Inc.
Rahway, New Jersey, United States 
Jiaying Liu, Ph.D. (she/her/hers)
Associate Principal Scientist
Merck & Co., Inc.
Kenilworth, New Jersey, United States- NK
Navin Kafle, Ph.D.
Senior scientist
Merck & Co., Inc.
Rahway, New Jersey, United States 
Tony Zhou, PhD (he/him/his)
Professor
Purdue University
West Lafayette, Indiana, United States- LQ
Li Qu, Ph.D.
Research Professor
Purdue University
West Lafayette, Indiana, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: Spray-dried formulations were prepared using Bovine Serum Albumin (BSA) as a model protein. Two formulation types were investigated: BSA with polymers or sugars (1:1 w/w ratio), and BSA with both polymers and sugars (1:1:1 w/w/w ratio), as detailed in Table 1. All formulations included sodium citrate buffer (20 mM), sodium chloride (150 mM), and polysorbate 80 (0.025% w/v). Spray drying was performed with an inlet temperature of 120 °C, outlet temperatures between 60–65 °C, a feed rate of 2 mL/min, and an atomization air flow of 600 L/h. The resulting powders underwent accelerated stability testing at 40 °C. Protein physical stability was assessed using size exclusion chromatography (SEC) and solid-state Fourier-transform infrared spectroscopy (ssFTIR). Powder characteristics were evaluated by scanning electron microscopy (SEM) and powder X-ray diffraction (PXRD).
Results: The physical stability of BSA at the end of 90-day accelerated storage showed that polymers such as hydrolyzed gelatin or (2-hydroxypropyl)-β-cyclodextrin (HPβCD) were able to significantly reduce the monomer loss (∼2-fold) compared to traditionally used protein stabilizer such as trehalose (Figure 1). While polymers like dextran (MW 20,000) or carboxymethyl cellulose sodium (NaCMC) were not able to stabilize BSA, as an increase in the monomer loss (∼2-fold) was observed compared to trehalose (Figure 1). The 2nd derivative ssFTIR result showed that NaCMC and mannitol formulations had perturbations in secondary structure of BSA at the end of 90-day accelerated storage (Figure 2). This study also revealed that combining hydrolyzed gelatin or HPβCD with trehalose or mannitol significantly decreased the monomer loss (∼2-5-fold) compared to using trehalose or mannitol alone (Figure 1). Powder characteristics such as residual moisture content, particle size and morphology were not found to be impacted by the use of different stabilizing excipients. These powder characteristics were not observed to impact stability. PXRD analysis revealed that all that the only formulation component that was crystallized was observed to be sodium chloride. None of the stabilizing polymers/ sugar-based excipients were observed to be crystallized.
Conclusion: This study suggested that polymers like hydrolyzed gelatin or HPβCD are a superior alternative to traditionally used stabilizer like trehalose. Both hydrolyzed gelatin or HPβCD were able to improve the physical stability of spray-dried BSA formulations during drying and upon storage. Furthermore, combining these polymers with trehalose or mannitol enhanced the stability of BSA compared to using trehalose or mannitol alone. In contrast, polymers such as sodium carboxymethyl cellulose (NaCMC) and dextran were ineffective under the tested conditions. This study also demonstrates that crystallization of sodium chloride did not impact the physical stability of BSA.
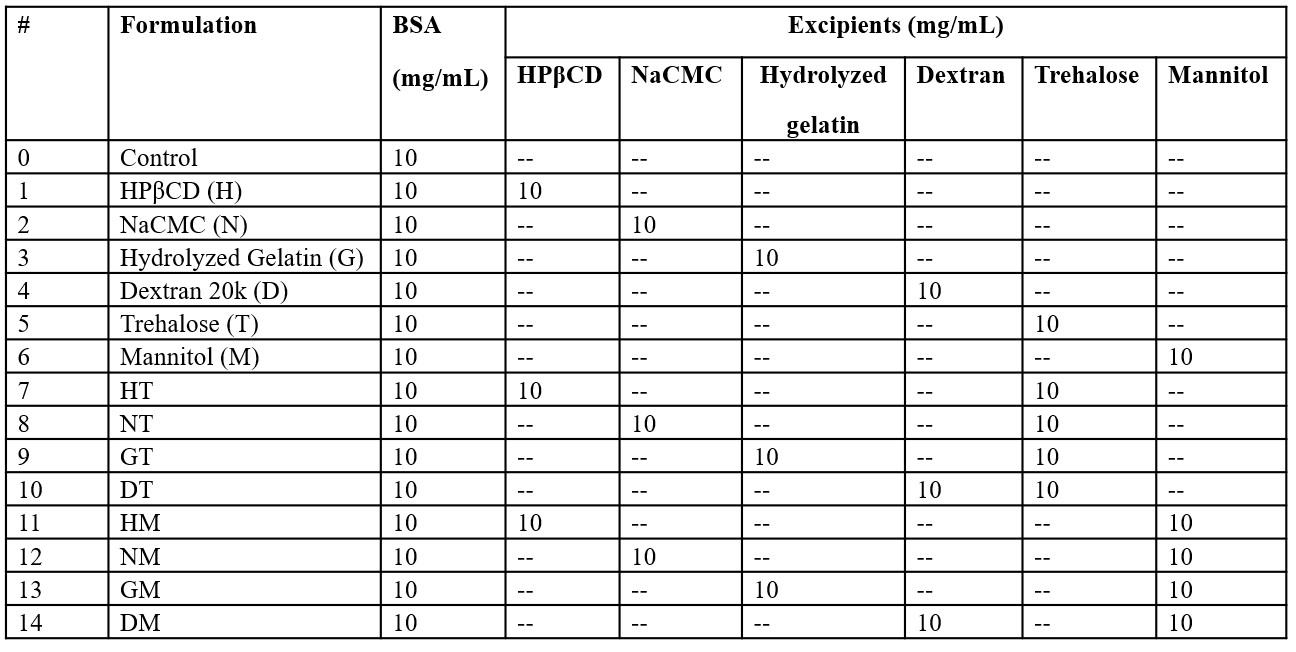 Table 1. Concentrations of protective excipients in the formulations.
Table 1. Concentrations of protective excipients in the formulations.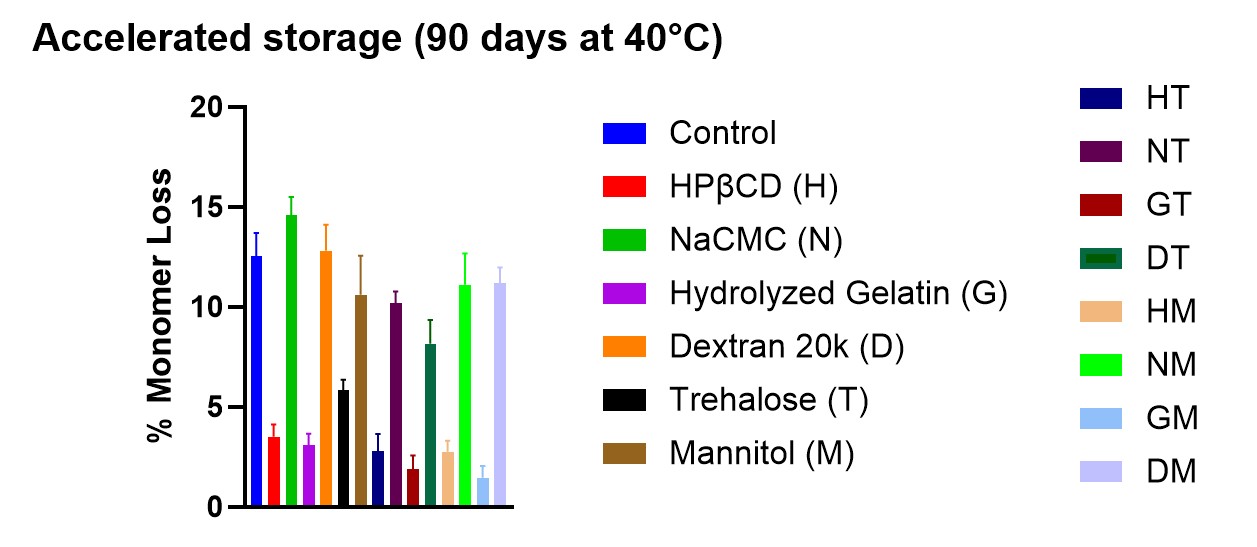 Figure 1. Monomer loss of BSA over 90 days for the samples stored under the accelerated condition at 40°C (n=5; Mean ± SD). *Refer to formulation details in Table 1.
Figure 1. Monomer loss of BSA over 90 days for the samples stored under the accelerated condition at 40°C (n=5; Mean ± SD). *Refer to formulation details in Table 1. 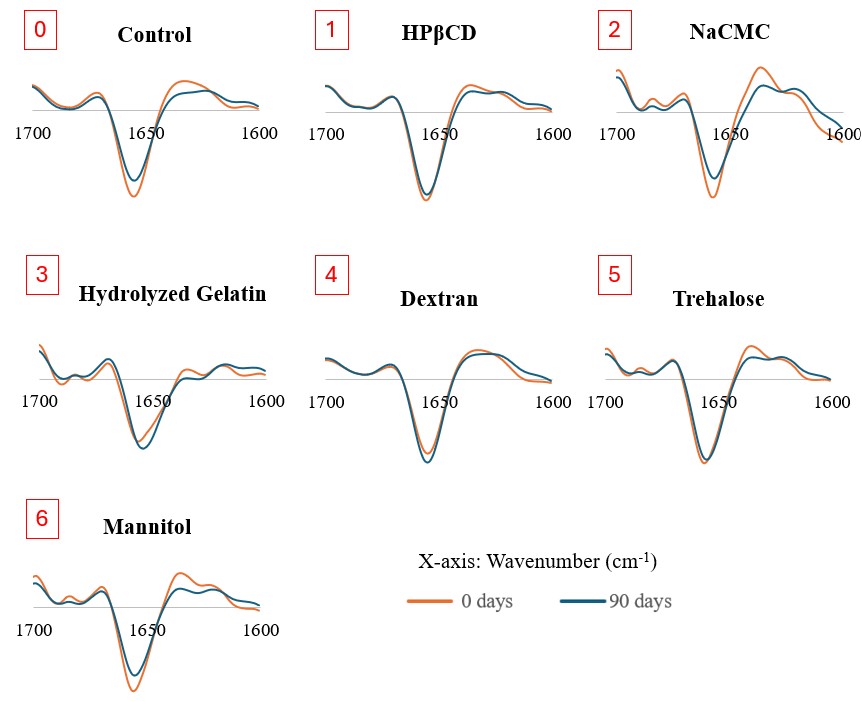 Figure 2. The second derivative ssFTIR spectra of the spray-dried formulations.
Figure 2. The second derivative ssFTIR spectra of the spray-dried formulations.