Formulation and Delivery - Chemical
(M1030-09-59) Evaluation of Spray Dried and Nanomilled Formulations on Pharmacokinetics Using Predictive Modeling

Nairuti Milan Mehta, MS
Scientist II, Research and Development
Thermo Fisher Scientific
Bend, Oregon, United States
Nairuti Milan Mehta, MS
Scientist II, Research and Development
Thermo Fisher Scientific
Bend, Oregon, United States- TR
Tom A. Reynolds, Ph.D., Ph.D.
Manager, Research and Development
Thermo Fisher Scientific
Bend, Oregon, United States 
Dineli T.S. T. S Ranathunga, Ph.D.
Scientist III, Research and Development
Thermo Fisher Scientific
Bend, Oregon, United States
James Mullin, M.S.
Senior Principal Scientist
Simulations Plus, Inc.
Durham, North Carolina, United States- TY
Thomas Yonker, BS, MBA
Vice President, Operations
Cerevance
Boston, Massachusetts, United States - SV
Sagar Vaidya, M.D., Ph.D
Chief Medical Officer
Cerevance
Boston, Massachusetts, United States 
Sanjay Konagurthu, Ph.D.
Sr. Director, Science and Innovation
Thermo Fisher Scientific
Morrisville, North Carolina, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: CVN424 is a low solubility Biopharmaceutics Classification System (BCS) Class II weak base. Validated mechanistic models were developed with the available preclinical data in animal species including rats, dogs and monkeys. The human model was built and validated using the available data from two primary human studies which included a dose ranging study for suspension and tablet formulations in fasted and fed states. Enterohepatic circulation was accounted for in the model to match the terminal phase secondary peaks by adjusting the biliary clearance fraction based on the data from the bile duct cannulated rat study. This validated mechanistic model was used to 1) evaluate the effect of dose and formulation technology in fasted and fed state and 2) predict the effect of meal type on the exposure of CVN424.
Results: Fig. 1 displays the observed vs simulated mean plasma concentration - time profiles of the microcrystalline API suspensions and tablet formulations in fasted and fed humans. The human model incorporated enterohepatic circulation by adjusting the biliary clearance fraction to 0.8 and allowing for reabsorption of the compound. A positive food effect was observed in the tablet formulation with a predicted fed/fasted Cmax ratio of 3.05 and an AUC ratio of 2.24. Sensitivity analysis was performed to look at the effect of dose and formulation technology on the exposure of CVN424 in fasted and fed states as shown in Fig 2. The amorphous solid dispersion (ASD) formulations assuming a 5x and 10x solubility enhancement ratio performed better in mitigating the food effect as compared to the current tablet and the optimized conventional tablet formulations (Fig. 2A - 2B). Additionally, the model predicted that the nanosuspension formulations could reduce the dose to 50 mg with no fed/fasted effect (Fig. 2C). To further evaluate the effect of fed meals on the absorption of CVN424, sensitivity analysis was performed on the 150 mg tablet formulation to determine and compare the effect of fat and calorie content of the meal as displayed in Fig 3. The model predicts that there is a strong effect of meal type with the Cmax increasing from 374.36 ng/mL for the low fat – low calorie meal to 1017.5 ng/mL for the high fat – high calorie meal.
Conclusion: The study effectively utilized mechanistic absorption and physiologically based pharmacokinetic (PBPK) modeling to evaluate the effects of dose and formulation technology on the absorption and exposure of CVN424. The results demonstrated a significant positive food effect with the API formulations, indicated by higher peak plasma concentrations and overall exposure in the fed state compared to the fasted state. Sensitivity analysis revealed amorphous solid dispersions and nanosuspensions were more effective in reducing the fed/fasted effect as compared to the conventional tablet formulations. Additionally, meal type significantly impacted drug absorption with higher fat and calorie meals increasing peak plasma concentrations and exposure. Overall, the predictive modeling approach provided valuable early insights into the performance of different formulations and food effect, supporting informed decision making and optimizing the drug development process.
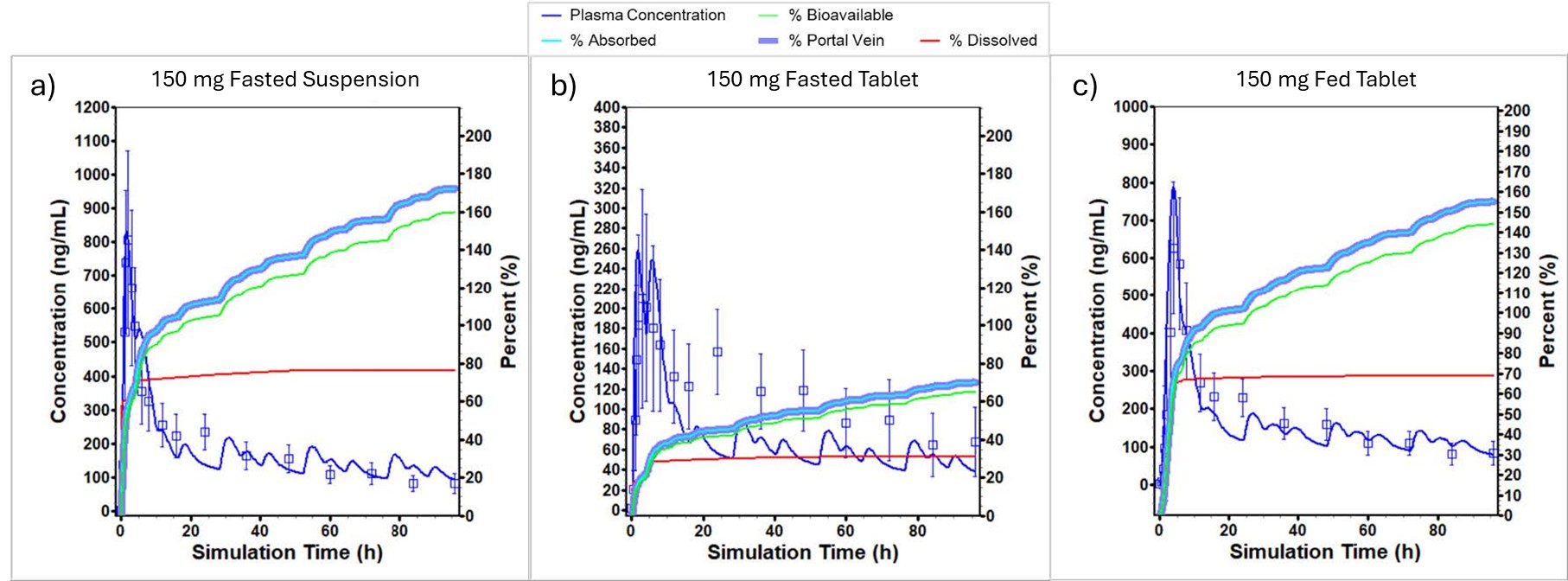 Figure 1: Observed and Simulated Results of a) 150 mg Oral Suspension, b) Fasted 150 mg Tablet, and c) Fed 150 mg Tablet in Human Study.
Figure 1: Observed and Simulated Results of a) 150 mg Oral Suspension, b) Fasted 150 mg Tablet, and c) Fed 150 mg Tablet in Human Study.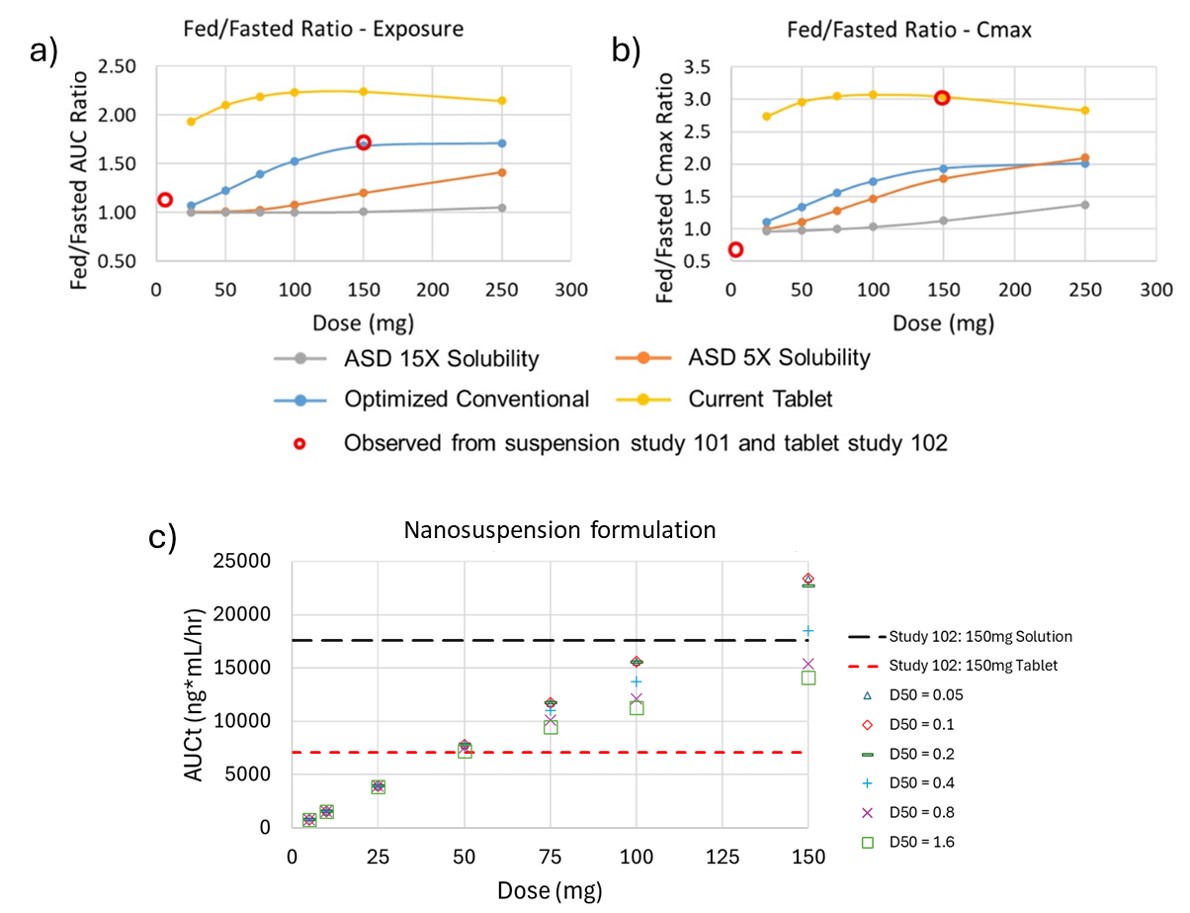 Figure 2: (a) Fed/Fasted Ratio of AUC to Dose and Formulation Type, (b) Fed/Fasted Ratio of Cmax to Dose and Formulation Type and (c) AUC vs Dose of Nanosuspension and API Formulations.
Figure 2: (a) Fed/Fasted Ratio of AUC to Dose and Formulation Type, (b) Fed/Fasted Ratio of Cmax to Dose and Formulation Type and (c) AUC vs Dose of Nanosuspension and API Formulations.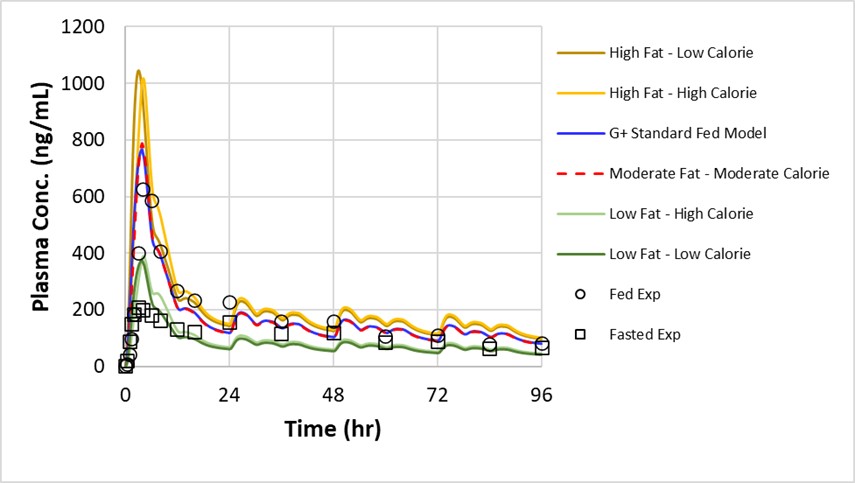 Figure 3: Sensitivity of Plasma Concentration to Meal Type for 150 mg Tablet of CVN424 in Human.
Figure 3: Sensitivity of Plasma Concentration to Meal Type for 150 mg Tablet of CVN424 in Human.