Formulation and Delivery - Biomolecular
(M1130-08-49) Understanding Stabilization Potential of Polymeric Excipients for Spray-Dried Monoclonal Antibody Formulations
- CP
Chanakya Deepak Patil, MS
Graduate Student
Purdue University
West Lafayette, Indiana, United States - CP
Chanakya Deepak Patil, MS
Graduate Student
Purdue University
West Lafayette, Indiana, United States 
Kinnari Santosh Arte, PhD (she/her/hers)
Scientist I
Alexion - AstraZeneca Rare Disease
Hamden, Connecticut, United States- RS
Rachana Sapkota, MS (she/her/hers)
Graduate Student
Purdue University
West Lafayette, Indiana, United States - YH
Yijing Huang, MS
Graduate Student
Purdue University
West Lafayette, Indiana, United States - HR
Harshil K Renawala, Ph.D.
Associate Principal Scientist
Merck & Co., Inc.
Rahway, New Jersey, United States 
Jiaying Liu, Ph.D. (she/her/hers)
Associate Principal Scientist
Merck & Co., Inc.
Kenilworth, New Jersey, United States- NK
Navin Kafle, Ph.D.
Senior scientist
Merck & Co., Inc.
Rahway, New Jersey, United States 
Eric J. Munson, PhD
Professor
Purdue University
West Lafayette, Indiana, United States
Tony Zhou, PhD (he/him/his)
Professor
Purdue University
West Lafayette, Indiana, United States- LQ
Li Qu, Ph.D.
Research Professor
Purdue University
West Lafayette, Indiana, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: Monoclonal antibody formulations were prepared with each excipient in a 1:1 (w/w) protein-to-excipient ratio and spray-dried using a Büchi B-290 spray dryer at 120 °C inlet and 60–65 °C outlet temperature. The dried powders were characterized for particle size distribution (Mastersizer), surface morphology (SEM), and residual moisture (Karl Fischer coulometry). Glass transition temperature (Tg) was assessed via differential scanning calorimetry (DSC). Physical stability was evaluated using size exclusion chromatography (SEC) for monomer content over 90 days of accelerated storage (40 °C). Secondary structure was analyzed by solid-state Fourier transform infrared spectroscopy (ssFTIR). Matrix miscibility was assessed using solid-state NMR, and particle surface composition was evaluated by X-ray photoelectron spectroscopy (XPS) to determine nitrogen (protein) and chlorine (NaCl) surface content.
Results: Spray drying did not induce significant monomer loss in any of the formulations at time 0, indicating mAb tolerance to process stress. After 90 days of accelerated storage, hydrolyzed gelatin demonstrated the most effective stabilization, with a five-fold reduction in monomer loss compared to trehalose (Figure 1). HPβCD showed comparable stabilization to trehalose but with lower protein surface exposure, likely due to its surface-active properties. PVP exhibited molecular weight-dependent effects: low molecular weight PVP K30 destabilized the mAb, while high molecular weight PVP K90 improved stability by 2.5-fold, attributed to enhanced matrix homogeneity and reduced surface protein exposure (Table 1). Mannitol crystallized during storage, resulting in high monomer loss and secondary structure perturbation (Figure 2). NaCMC maintained secondary structure but showed increased aggregation, possibly due to charge interactions. Dextran and HPMC failed to stabilize mAb formulations under accelerated storage conditions. XPS data confirmed that HPβCD and high molecular weight PVP reduced protein surface exposure compared to the control and other excipients. Despite high Tg values ( >90 °C), protein stability correlated more closely with matrix homogeneity and surface behavior than with Tg alone.
Conclusion: This study demonstrates that the stabilization of spray-dried mAb formulations is influenced by a complex interplay of factors, including matrix homogeneity, polymer molecular weight, surface activity, and crystallization behavior. Hydrolyzed gelatin and HPβCD emerged as promising stabilizers, with high molecular weight PVP also providing significant protection. These findings suggest that excipient selection for spray-dried biologics should consider both molecular interactions and processing-related stressors.
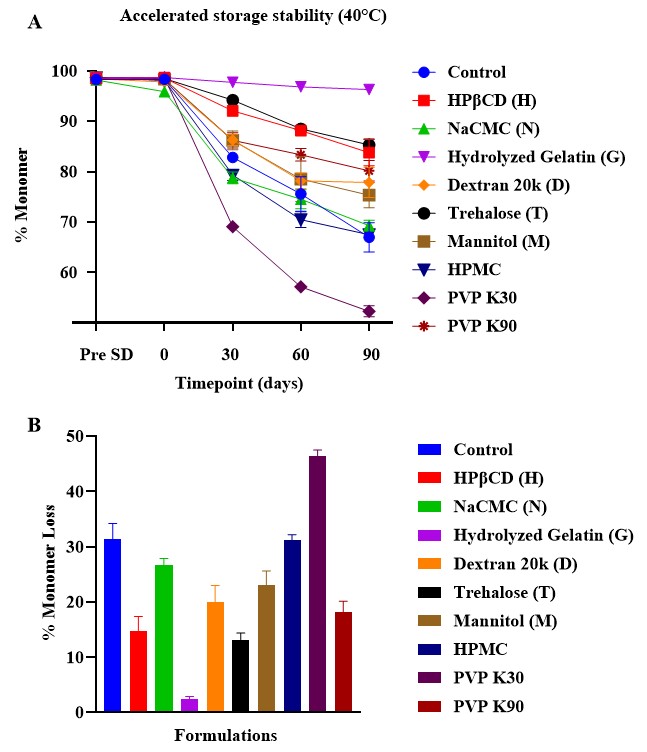 Figure 1. A: % monomer of mAb at each accelerated stability timepoint, B: % monomer loss of mAb over 90 days for the samples stored under the accelerated condition at 40°C (n=5; Mean ± SD).
Figure 1. A: % monomer of mAb at each accelerated stability timepoint, B: % monomer loss of mAb over 90 days for the samples stored under the accelerated condition at 40°C (n=5; Mean ± SD).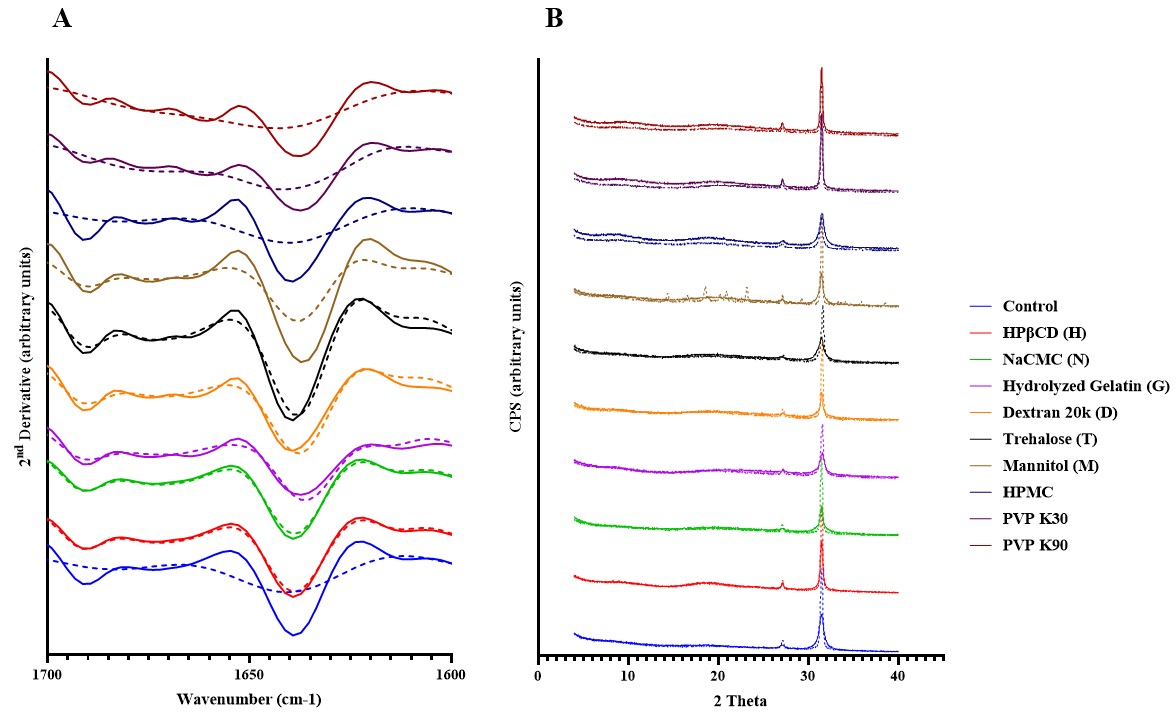 Figure 2. A: The 2nd derivative ssFTIR spectra of the formulations. B: X-ray diffractograms for the formulations. Solid line represents T=0 samples; dashed line represents samples at 90 days of accelerated storage.
Figure 2. A: The 2nd derivative ssFTIR spectra of the formulations. B: X-ray diffractograms for the formulations. Solid line represents T=0 samples; dashed line represents samples at 90 days of accelerated storage.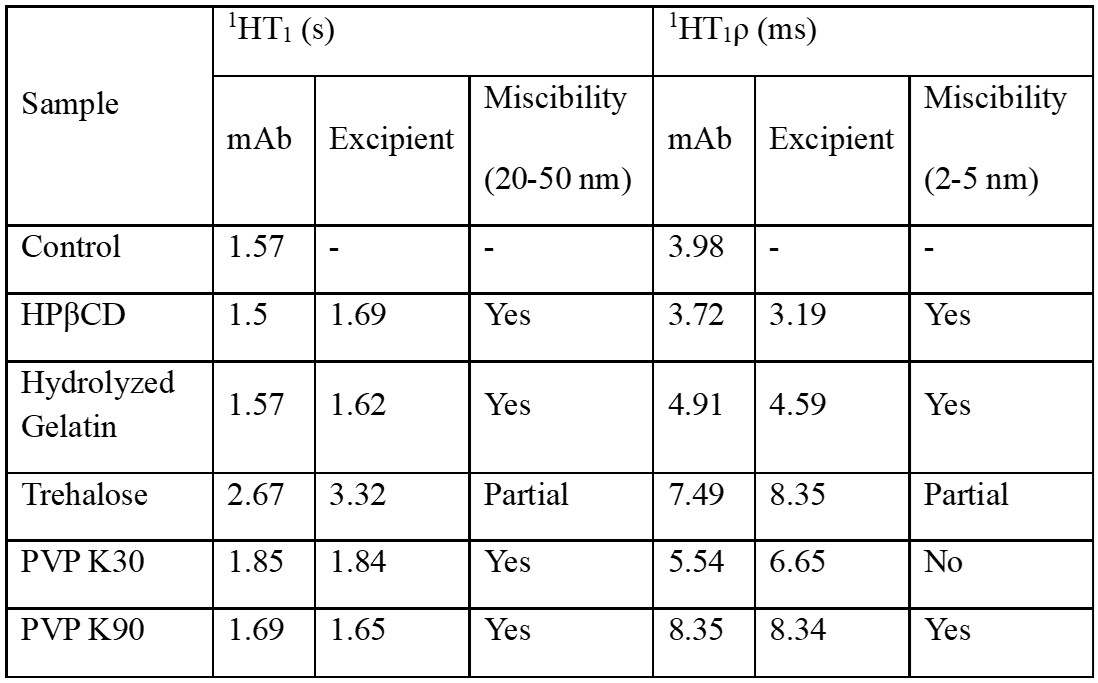 Table 1: Summary of solid state- NMR and miscibility of monoclonal antibody (mAb), and each stabilizing excipient used in the spray-dried formulations.
Table 1: Summary of solid state- NMR and miscibility of monoclonal antibody (mAb), and each stabilizing excipient used in the spray-dried formulations.