Manufacturing and Analytical Characterization - Chemical
(M1230-02-11) Synergistic Integration of Twin-Screw Hot-Melt Granulation and Selective Laser Melting for High-Dose Extended-Release 3D-Printed Tablets
Monday, November 10, 2025
12:30 PM - 1:30 PM CT

Bhupendra Raj Giri, MS
Graduate Student
University of Texas at Austin
Austin, Texas, United States
Bhupendra Raj Giri, MS
Graduate Student
University of Texas at Austin
Austin, Texas, United States
Abdelrahman M. Helmy, PhD (he/him/his)
Assistant Professor
Badr University in Assiut
Asyut, Asyut, Egypt- MM
Mohammed Maniruzzaman, Ph.D.
Professor
University of Mississippi
Oxford, Mississippi, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Over the years, the rapid growth of three-dimensional printing (3DP) has offered transformative potential for pharmaceutical manufacturing, enabling patient-specific dosage and unique drug delivery systems that are difficult to achieve with traditional pharmaceutical technologies [1,2]. Powder-based methods such as selective laser sintering (SLS) are particularly attractive as they use powder as feedstock materials, and active pharmaceutical ingredients (APIs) and excipients are mostly available as powders [3]. However, challenges such as poor and uneven powder flow and differences in thermal characteristics of APIs and excipients result in inefficient sintering, leading to high porosity and low mechanical strength in 3D-printed tablets [4]. Additionally, such tablets tend to have a low drug load, disintegrate, and release the drug within a few minutes, which limits their application for prolonged-release dosage forms. To address these limitations, we investigated an alternative powder bed fusion technique, selective laser melting (SLM), which fully melts and fuses materials to create dense and robust structures. SLM was coupled with a hot-melt extruder (HME) to prepare lipid-based drug-loaded granular feedstock with optimal flow properties and thermal characteristics for efficient fusion. Thus, this novel approach aims to overcome the limitations of conventional SLS 3DP by fabricating low-porosity, high-drug-load, and high-tensile-strength 3D-printed tablets capable of achieving an extended-release profile.
Methods: Acetaminophen (ACM) and lipid (Compritol® 888 ATO) physical mixtures with varying drug loads (55–85% w/w) were processed using a twin-screw HME to produce granules (HMG-55 to HMG-85), which were then milled and sieved to a target particle size suitable for 3DP. The particle size distribution (PSD) of the granules was determined, and bulk and tapped densities, Carr's Index (CI), and Hausner's Ratio (HR) were calculated to evaluate powder flowability. This physical mixture (PM) was then blended with Candurin®, 1.5% w/w, to aid the melting process. A benchtop SLS 3D printer was used to fabricate tablets, with key printing parameters optimized for a 150 µm layer thickness, 50 mm/s laser speed, and 65°C print surface temperature. The solid state of the API, carriers, and HMGs was assessed using DSC, PXRD, SEM, PLM, X-ray micro-CT, and digital light microscopy. A non-pH shift dissolution study was performed to compare drug release profiles from different SLM 3D-printed tablets.
Results: The neat drug, lipid, and their physical mixture showed very poor flow properties (CI > 39), while the flow properties were improved after granulation, particularly for formulations with high lipid levels, i.e., HMG55 and HMG65, with drug-to-lipid ratios of 55:45 and 65:35, respectively. Additionally, increasing the HME screw speed (from 25 to 100 RPM) reduced the fraction of oversized granules. Conversely, increasing the number of heated zones in HME above the lipid’s melting point leads to oversized granules (Figure 1). ACM-loaded tablets with a high drug load (55-85% w/w, F-55 to F-85) were successfully printed (Figure 2) with optimal processing conditions: a screw speed of 75 rpm and an extrusion with a 4-heated-zone temperature profile (TP-4). Formulations with ≥25% w/w lipid (F-55, F-65, and F-75) yielded dense, high-tensile-strength tablets, while low-lipid formulations (F-85 with 15% w/w lipid) resulted in high weight variations and physical defects. Solid-state characterizations of HMGs showed that the drug remained in its native crystalline state, with no degradation during both the HME and SLM processes (Figure 2). The tablets with the highest drug-to-lipid load (F85) demonstrated nearly complete drug release within 24 h, whereas the F-55 formulation demonstrated an extended drug release profile of 12.2% over 24 h, attributed to the complete drug fusion within the lipid matrix, resulting in robust tablet formation (Figure 3). The drug was released mainly from the top and bottom surfaces, possibly due to weak sintering at these boundary surfaces. Kinetic modeling indicates that the drug release from the printed tablets is best described by the Higuchi model (R² ≥ 0.98), indicating diffusion-controlled release from a high-concentration drug within tablet pores.
Conclusion: The study focuses on the preparation of suitable SLM feedstock through hot melt granulation, focusing on key formulation and process parameters such as lipid content, screw speed, and temperature profile. The tablets fabricated via SLM of HMGs showed desirable mechanical strength, and a controlled drug release profile was found to be directly dependent on the drug-to-lipid ratio. This approach offers high flexibility, simplicity in printing solid oral dosage forms directly from the powder feedstock, and control over release properties, making it a first step towards high drug-load pharmaceutical formulations. Overall, our work demonstrated a first step towards the coupling of twin-screw hot-melt granulation and SLM 3DP techniques to enable high drug-load pharmaceutical formulations with extended drug release characteristics. This novel approach offers high flexibility, simplicity of printing solid oral dosage forms directly from the powder feedstock, and control over the drug release properties.
References: 1. Kulkarni VR, Saha T, Raj Giri B, Lu A, Das SC, Maniruzzaman M. Recent Advancements in Pharmaceutical 3D Printing Industry. J Drug Deliv Sci Technol [Internet]. 2024 [cited 2025 Jul 22];100:106072. Available from: https://www.sciencedirect.com/science/article/pii/S177322472400741X
2. Giri BR, Poudel S, Kim DW. Cellulose and its derivatives for application in 3D printing of pharmaceuticals. J Pharm Investig [Internet]. 2021;51:1–22. Available from: https://link.springer.com/10.1007/s40005-020-00498-5
3. Giri BR, Maniruzzaman M. Fabrication of Sustained-Release Dosages Using Powder-Based Three-Dimensional (3D) Printing Technology. AAPS PharmSciTech [Internet]. 2023 [cited 2025 Jul 22];24:1–15. Available from: https://link.springer.com/article/10.1208/s12249-022-02461-z
4. Davis DA, Thakkar R, Su Y, Williams RO, Maniruzzaman M. Selective Laser Sintering 3-Dimensional Printing as a Single Step Process to Prepare Amorphous Solid Dispersion Dosage Forms for Improved Solubility and Dissolution Rate. J Pharm Sci. 2021;110:1432–43.
5. Thakkar R, Zhang Y, Zhang J, Maniruzzaman M. Synergistic application of twin-screw granulation and selective laser sintering 3D printing for the development of pharmaceutical dosage forms with enhanced dissolution rates and physical properties. European Journal of Pharmaceutics and Biopharmaceutics [Internet]. 2021 [cited 2022 Oct 6];163:141–56. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0939641121000837
6. Thakkar R, Davis DA, Williams RO, Maniruzzaman M. Selective Laser Sintering of a Photosensitive Drug: Impact of Processing and Formulation Parameters on Degradation, Solid State, and Quality of 3D-Printed Dosage Forms. Mol Pharm [Internet]. 2021 [cited 2023 Apr 16];18:3894–908. Available from: https://pubmed.ncbi.nlm.nih.gov/34529431/
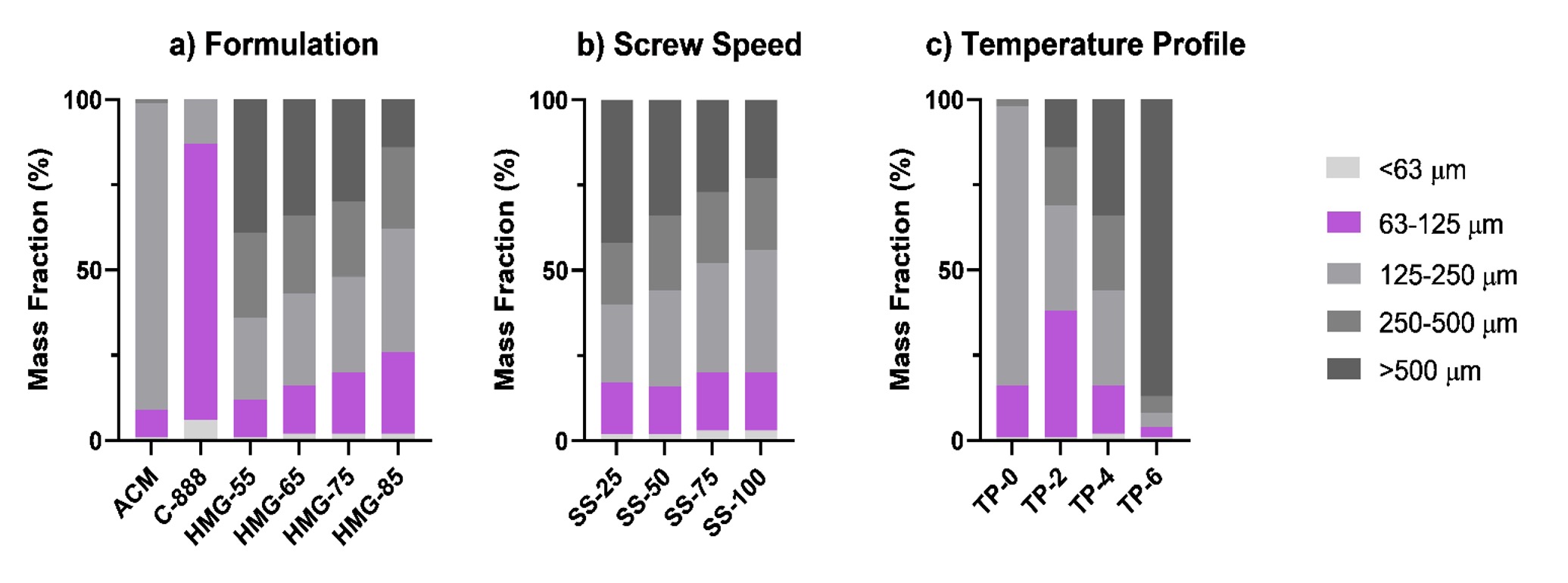 Figure 1. Mass fraction (%w/w) of hot-melt granules as a function of a) formulation ratios, b) screw speed, and c) extruder temperature profiles.
Figure 1. Mass fraction (%w/w) of hot-melt granules as a function of a) formulation ratios, b) screw speed, and c) extruder temperature profiles.
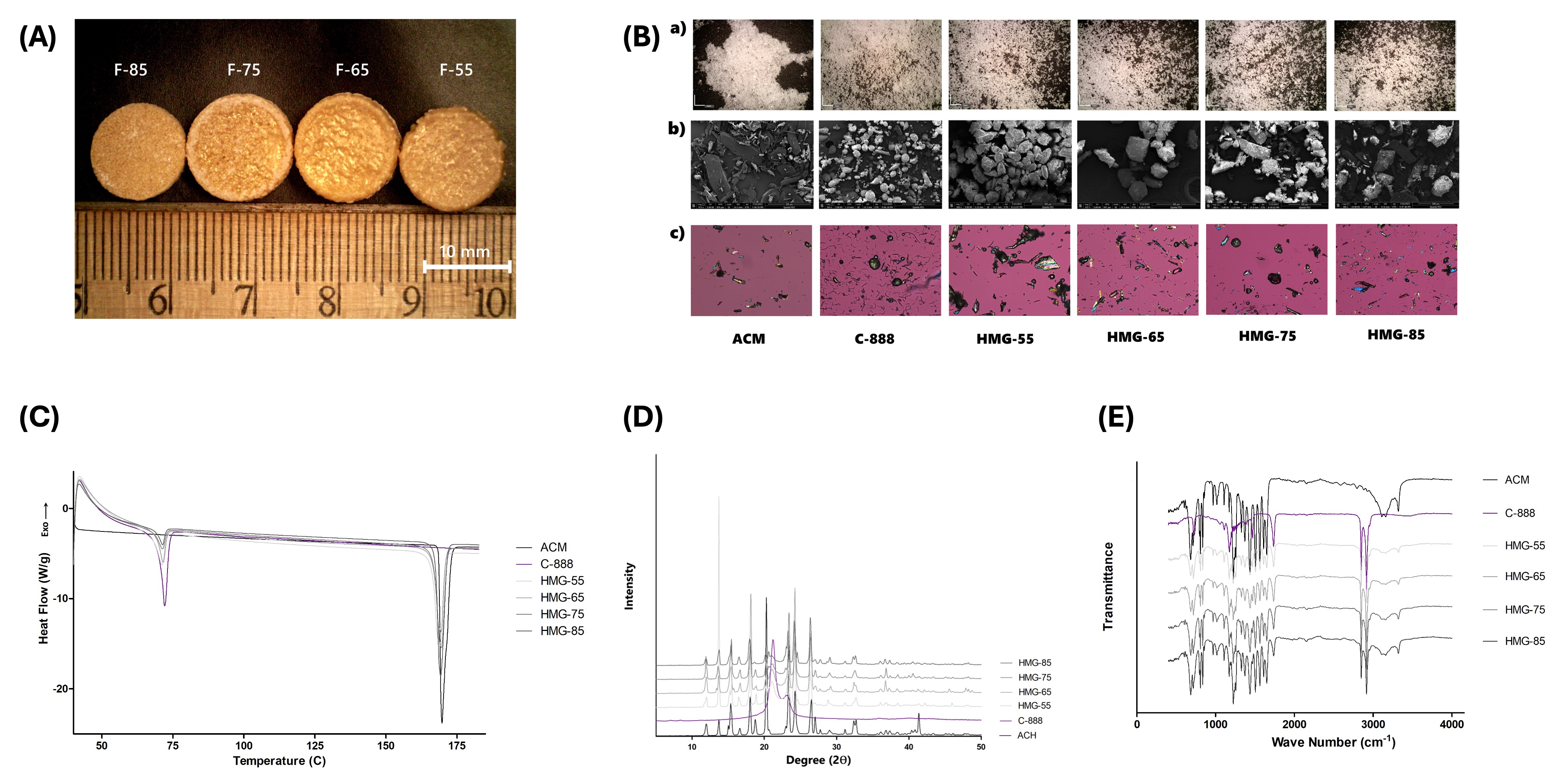 Figure 2: A) Digital light microscopic images of the SLM 3D-printed tablets with varying drug load; B) Digital light microscopic, scanning electron microscopy (SEM), and polarized light microscopy (PLM) images; C) differential scanning calorimetry; D) powder X-ray diffraction; and E) FT-IR spectra of the drug (ACM), lipid (C-888), and HMG granules from four different formulations denoted as HMG-55, HMG-65, HMG-75, and HMG-85 based on the 55-85% (w/w) drug load.
Figure 2: A) Digital light microscopic images of the SLM 3D-printed tablets with varying drug load; B) Digital light microscopic, scanning electron microscopy (SEM), and polarized light microscopy (PLM) images; C) differential scanning calorimetry; D) powder X-ray diffraction; and E) FT-IR spectra of the drug (ACM), lipid (C-888), and HMG granules from four different formulations denoted as HMG-55, HMG-65, HMG-75, and HMG-85 based on the 55-85% (w/w) drug load.
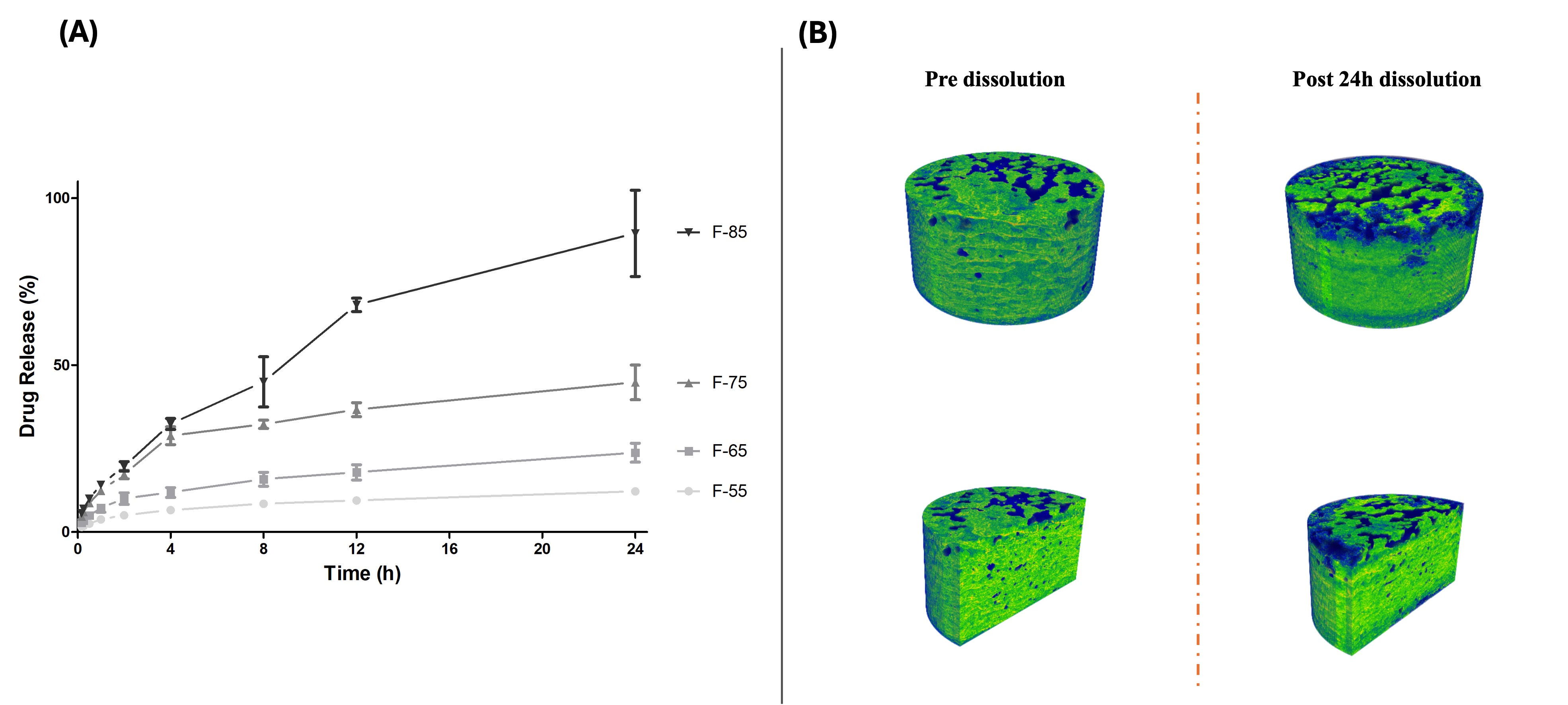 Figure 3: A) In vitro drug release profile of the SLM 3D-printed tablets from various formulations with varying drug loads (55–85% w/w); B) X-ray micro-CT images showing the top surface and inner pores of the SLM 3D-printed tablet (F-65 formulation) pre- and post-24 hours of dissolution study.
Figure 3: A) In vitro drug release profile of the SLM 3D-printed tablets from various formulations with varying drug loads (55–85% w/w); B) X-ray micro-CT images showing the top surface and inner pores of the SLM 3D-printed tablet (F-65 formulation) pre- and post-24 hours of dissolution study.
Methods: Acetaminophen (ACM) and lipid (Compritol® 888 ATO) physical mixtures with varying drug loads (55–85% w/w) were processed using a twin-screw HME to produce granules (HMG-55 to HMG-85), which were then milled and sieved to a target particle size suitable for 3DP. The particle size distribution (PSD) of the granules was determined, and bulk and tapped densities, Carr's Index (CI), and Hausner's Ratio (HR) were calculated to evaluate powder flowability. This physical mixture (PM) was then blended with Candurin®, 1.5% w/w, to aid the melting process. A benchtop SLS 3D printer was used to fabricate tablets, with key printing parameters optimized for a 150 µm layer thickness, 50 mm/s laser speed, and 65°C print surface temperature. The solid state of the API, carriers, and HMGs was assessed using DSC, PXRD, SEM, PLM, X-ray micro-CT, and digital light microscopy. A non-pH shift dissolution study was performed to compare drug release profiles from different SLM 3D-printed tablets.
Results: The neat drug, lipid, and their physical mixture showed very poor flow properties (CI > 39), while the flow properties were improved after granulation, particularly for formulations with high lipid levels, i.e., HMG55 and HMG65, with drug-to-lipid ratios of 55:45 and 65:35, respectively. Additionally, increasing the HME screw speed (from 25 to 100 RPM) reduced the fraction of oversized granules. Conversely, increasing the number of heated zones in HME above the lipid’s melting point leads to oversized granules (Figure 1). ACM-loaded tablets with a high drug load (55-85% w/w, F-55 to F-85) were successfully printed (Figure 2) with optimal processing conditions: a screw speed of 75 rpm and an extrusion with a 4-heated-zone temperature profile (TP-4). Formulations with ≥25% w/w lipid (F-55, F-65, and F-75) yielded dense, high-tensile-strength tablets, while low-lipid formulations (F-85 with 15% w/w lipid) resulted in high weight variations and physical defects. Solid-state characterizations of HMGs showed that the drug remained in its native crystalline state, with no degradation during both the HME and SLM processes (Figure 2). The tablets with the highest drug-to-lipid load (F85) demonstrated nearly complete drug release within 24 h, whereas the F-55 formulation demonstrated an extended drug release profile of 12.2% over 24 h, attributed to the complete drug fusion within the lipid matrix, resulting in robust tablet formation (Figure 3). The drug was released mainly from the top and bottom surfaces, possibly due to weak sintering at these boundary surfaces. Kinetic modeling indicates that the drug release from the printed tablets is best described by the Higuchi model (R² ≥ 0.98), indicating diffusion-controlled release from a high-concentration drug within tablet pores.
Conclusion: The study focuses on the preparation of suitable SLM feedstock through hot melt granulation, focusing on key formulation and process parameters such as lipid content, screw speed, and temperature profile. The tablets fabricated via SLM of HMGs showed desirable mechanical strength, and a controlled drug release profile was found to be directly dependent on the drug-to-lipid ratio. This approach offers high flexibility, simplicity in printing solid oral dosage forms directly from the powder feedstock, and control over release properties, making it a first step towards high drug-load pharmaceutical formulations. Overall, our work demonstrated a first step towards the coupling of twin-screw hot-melt granulation and SLM 3DP techniques to enable high drug-load pharmaceutical formulations with extended drug release characteristics. This novel approach offers high flexibility, simplicity of printing solid oral dosage forms directly from the powder feedstock, and control over the drug release properties.
References: 1. Kulkarni VR, Saha T, Raj Giri B, Lu A, Das SC, Maniruzzaman M. Recent Advancements in Pharmaceutical 3D Printing Industry. J Drug Deliv Sci Technol [Internet]. 2024 [cited 2025 Jul 22];100:106072. Available from: https://www.sciencedirect.com/science/article/pii/S177322472400741X
2. Giri BR, Poudel S, Kim DW. Cellulose and its derivatives for application in 3D printing of pharmaceuticals. J Pharm Investig [Internet]. 2021;51:1–22. Available from: https://link.springer.com/10.1007/s40005-020-00498-5
3. Giri BR, Maniruzzaman M. Fabrication of Sustained-Release Dosages Using Powder-Based Three-Dimensional (3D) Printing Technology. AAPS PharmSciTech [Internet]. 2023 [cited 2025 Jul 22];24:1–15. Available from: https://link.springer.com/article/10.1208/s12249-022-02461-z
4. Davis DA, Thakkar R, Su Y, Williams RO, Maniruzzaman M. Selective Laser Sintering 3-Dimensional Printing as a Single Step Process to Prepare Amorphous Solid Dispersion Dosage Forms for Improved Solubility and Dissolution Rate. J Pharm Sci. 2021;110:1432–43.
5. Thakkar R, Zhang Y, Zhang J, Maniruzzaman M. Synergistic application of twin-screw granulation and selective laser sintering 3D printing for the development of pharmaceutical dosage forms with enhanced dissolution rates and physical properties. European Journal of Pharmaceutics and Biopharmaceutics [Internet]. 2021 [cited 2022 Oct 6];163:141–56. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0939641121000837
6. Thakkar R, Davis DA, Williams RO, Maniruzzaman M. Selective Laser Sintering of a Photosensitive Drug: Impact of Processing and Formulation Parameters on Degradation, Solid State, and Quality of 3D-Printed Dosage Forms. Mol Pharm [Internet]. 2021 [cited 2023 Apr 16];18:3894–908. Available from: https://pubmed.ncbi.nlm.nih.gov/34529431/
 Figure 1. Mass fraction (%w/w) of hot-melt granules as a function of a) formulation ratios, b) screw speed, and c) extruder temperature profiles.
Figure 1. Mass fraction (%w/w) of hot-melt granules as a function of a) formulation ratios, b) screw speed, and c) extruder temperature profiles. Figure 2: A) Digital light microscopic images of the SLM 3D-printed tablets with varying drug load; B) Digital light microscopic, scanning electron microscopy (SEM), and polarized light microscopy (PLM) images; C) differential scanning calorimetry; D) powder X-ray diffraction; and E) FT-IR spectra of the drug (ACM), lipid (C-888), and HMG granules from four different formulations denoted as HMG-55, HMG-65, HMG-75, and HMG-85 based on the 55-85% (w/w) drug load.
Figure 2: A) Digital light microscopic images of the SLM 3D-printed tablets with varying drug load; B) Digital light microscopic, scanning electron microscopy (SEM), and polarized light microscopy (PLM) images; C) differential scanning calorimetry; D) powder X-ray diffraction; and E) FT-IR spectra of the drug (ACM), lipid (C-888), and HMG granules from four different formulations denoted as HMG-55, HMG-65, HMG-75, and HMG-85 based on the 55-85% (w/w) drug load. Figure 3: A) In vitro drug release profile of the SLM 3D-printed tablets from various formulations with varying drug loads (55–85% w/w); B) X-ray micro-CT images showing the top surface and inner pores of the SLM 3D-printed tablet (F-65 formulation) pre- and post-24 hours of dissolution study.
Figure 3: A) In vitro drug release profile of the SLM 3D-printed tablets from various formulations with varying drug loads (55–85% w/w); B) X-ray micro-CT images showing the top surface and inner pores of the SLM 3D-printed tablet (F-65 formulation) pre- and post-24 hours of dissolution study.