Preclinical, Clinical, and Translational Sciences
(M1330-05-31) Local Delivery of Recombinant Human CST6 Protein and Human CST6-Coding mRNA for Bone Regeneration
- PP
Pornpoj Phruttiwanichakun, Pharm.D., M.Sc. (they/them/theirs)
Graduate student
University of Iowa
Iowa City, Iowa, United States - PP
Pornpoj Phruttiwanichakun, Pharm.D., M.Sc. (they/them/theirs)
Graduate student
University of Iowa
Iowa City, Iowa, United States - RH
Rui He, MS
Graduate Student
University of Iowa
Iowa City, Iowa, United States - DF
Douglas C. Fredericks (they/them/theirs)
Animal Research Surgicenter Director
University of Iowa
Coralville, Iowa, United States - EP
Emily B. Petersen
Animal Research Surgicenter Head Veterinarian
University of Iowa
Coralville, Iowa, United States - SP
Sainiket Panyam
Scholar/Trainee
University of Iowa
Iowa City, Iowa, United States 
Aliasger K. Salem, PhD
Bighley Chair and Professor of Pharmaceutical Sciences
University of Iowa
Iowa City, Iowa, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: rhCST6 protein was investigated for its osteogenic and matrix mineralization effects in human bone marrow-derived mesenchymal stem cells (hBMSCs), and in murine pre-osteoblast MC3T3-E1 subclone 4 cell line. For osteogenic effects, hBMSCs and MC3T3-E1 cells were treated with various concentrations of rhCST6 protein in an osteo-permissive (OP; for hBMSCs) or in an osteogenic medium (OM; for MC3T3-E1) for 7 days before cell lysate harvest for alkaline phosphatase activity and total dsDNA assays. For matrix mineralization, MC3T3-E1 cells were treated in a similar fashion for 21 days before paraformaldehyde fixation and alizarin red S staining for calcium deposits. Chemically modified mRNA that encodes rhCST6 protein or encodes rhCST6-P2A-eGFP fusion protein (eGFP as the reporter protein; P2A as the self-cleavable sequence) was in vitro transcribed (with 100% N1-methyl pseudouridine replacement) and encapsulated in SM102-based lipid nanoparticles (LNPs) using microfluidic mixing. The effect of the LNPs on cell viability was tested using MTS assays at 24 hours after delivering various doses of LNPs to murine macrophage-like RAW264.7 cells, hBMSCs, and MC3T3-E1 cells. The functionality of the LNPs was also tested by quantifying the conditioned medium of hBMSCs and MC3T3-E1 cells after LNP delivery for secreted rhCST6 protein levels using ELISA. To assess the in vivo effects of rhCST6 protein and CST6-encoding mRNA (as LNPs) on bone healing, a metaphyseal drill hole defect model was utilized by creating the defect at the medial femoral metaphysis of male and female 17-20 weeks old Sprague-Dawley rats. The rhCST6 protein (15-µg protein equivalence per plug) or the CST6 LNPs (25-µg mRNA equivalence per plug) were lyophilized onto collagen type I plugs before insertion into the drill hole defects and healing was evaluated at 6 weeks after surgery using micro-CT and histology.
Results: rhCST6 protein promoted osteogenic differentiation of hBMSCs in the presence of OP media as evidenced by significantly higher levels of total dsDNA-normalized alkaline phosphatase activity in the cell lysates after 7 days of rhCST6 treatment compared to the OP medium control (Figure 1a). Under the same treatment conditions, rhCST6 protein also promoted hBMSC proliferation as shown by significant increases in total dsDNA contents compared to the OP medium control (Figure 1b). rhCST6 significantly promoted matrix mineralization in MC3T3-E1 subclone 4 monolayers as reflected by a significant increase in alizarin red S staining after 21 days of treatment (measured via acetic acid extraction and spectrophotometry; Figure 1c). CST6-P2A-eGFP LNP delivery showed minimal cytotoxicity in RAW264.7, MC3T3-E1, and hBMSCs Figure 2a-c). Expression of rhCST6 from transfected MC3T3-E1 and hBMSCs was demonstrated by the expression of the eGFP reporter (Figure 2d-e) using flow cytometry and confirmed in the supernatant using ELISA (Figure 2f), yielding nanogram-per-mL levels of secreted rhCST6 . Finally, for an in vivo study, the CST6-LNP group showed no significant differences compared to the lyophilized excipient control (LA) in all measurements (Figure 3) on week 6 after implantation. In contrast, the lyophilized rhCST6 protein (15-µg protein) group showed a significant increase in relative bone volume in the defect region-of-interest (Figure 3b) compared to the LA group.
Conclusion: Our study demonstrated the potential of rhCST6 protein as a novel osteogenic protein. In summary, rhCST6 protein was shown to promote osteogenic differentiation and mineralization in vitro, and to promote bone regeneration in vivo, paving ways for further formulation development of local delivery of rhCST6 protein for better bone healing. However, its CST6-encoding mRNA (as LNPs) counterpart failed to significantly promote bone regeneration in vivo, signifying the need for further investigation and optimization of the LNP formulation, the mRNA dose, and the method of local LNP delivery.
References: 1. Gai D, Chen JR, Stewart JP, Nookaew I, Habelhah H, Ashby C, Sun F, Cheng Y, Li C, Xu H, Peng B, Garg TK, Schinke C, Thanendrarajan S, Zangari M, Chen F, Barlogie B, van Rhee F, Tricot G, Shaughnessy JD Jr, Zhan F. CST6 suppresses osteolytic bone disease in multiple myeloma by blocking osteoclast differentiation. J Clin Invest. 2022 Sep 15;132(18):e159527.
2. Li X, Liang Y, Lian C, Peng F, Xiao Y, He Y, Ma C, Wang Y, Zhang P, Deng Y, Su Y, Luo C, Kong X, Yang Q, Liu T, Hu G. CST6 protein and peptides inhibit breast cancer bone metastasis by suppressing CTSB activity and osteoclastogenesis. Theranostics. 2021 Oct 11;11(20):9821-9832.
Acknowledgements: The authors are grateful for the financial support from the NIH (5R21DE031042-02 , under NIDCR). PP acknowledges the support of the Keith Guillory Pharmaceutics Fellowship and the University of Iowa Center for Biocatalysis and Bioprocessing Predoctoral Fellowship in Biotechnology. RH acknowledges the support of the UI Pharmaceuticals Fellowship. SP acknowledges the support of the Belin-Blank Center through the Secondary Student Training Program. PP and RH would also like to acknowledge the guidance and the support from Dr. Aliasger K. Salem, Dr. Sean M. Geary, Dr. Douglas C. Fredericks, Dr. Emily B. Petersen, Dr. James Martin, Dr. Dong Rim Seol, Dr. Alexandra McMillan, and Dr. Noah Z. Laird.
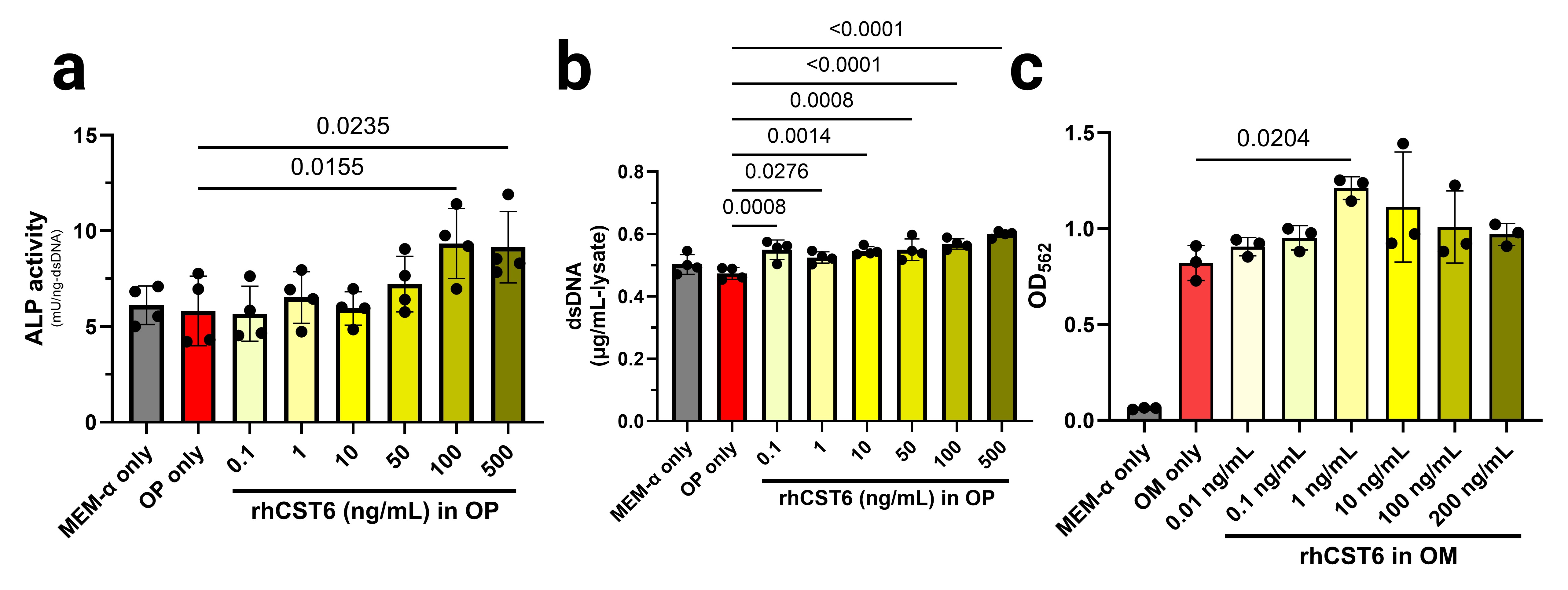 Figure 1: In vitro osteogenic and mineralization effects of rhCST6 protein in hBMSCs and MC3T3-E1 (subclone 4) cells in presence of osteo-permissive or osteogenic media. (a) alkaline phosphatase activity assay of hBMSC cell lysates with normalization to the total dsDNA content in the lysate (after 7 days of culture). (b) total dsDNA contents in the hBMSC cell lysates (after 7 days of culture). (c) semi-quantification of alizarin red S staining of the MC3T3-E1 cell monolayers with/without rhCST6 protein (after 7 days of culture). The alizarin red S-stained deposits were extracted using acetic acid and subsequently neutralized using ammonium hydroxide before absorbance measurements. MEM-α = complete MEM-α supplemented with 10% fetal bovine serum, 100 U/mL PenStrep, and 4 mM GlutaMAX™; OP = osteo-permissive medium; OM = osteogenic medium. OP and OM were of the same composition, which was the complete MEM-α supplemented with 10 mM β-glycerophosphate and 50 µg/mL magnesium ascorbyl phosphate. Statistical analysis was performed using ordinary one-way ANOVA followed by Dunnett’s tests versus the OP (or the OM) only group at the significance level of 0.05.
Figure 1: In vitro osteogenic and mineralization effects of rhCST6 protein in hBMSCs and MC3T3-E1 (subclone 4) cells in presence of osteo-permissive or osteogenic media. (a) alkaline phosphatase activity assay of hBMSC cell lysates with normalization to the total dsDNA content in the lysate (after 7 days of culture). (b) total dsDNA contents in the hBMSC cell lysates (after 7 days of culture). (c) semi-quantification of alizarin red S staining of the MC3T3-E1 cell monolayers with/without rhCST6 protein (after 7 days of culture). The alizarin red S-stained deposits were extracted using acetic acid and subsequently neutralized using ammonium hydroxide before absorbance measurements. MEM-α = complete MEM-α supplemented with 10% fetal bovine serum, 100 U/mL PenStrep, and 4 mM GlutaMAX™; OP = osteo-permissive medium; OM = osteogenic medium. OP and OM were of the same composition, which was the complete MEM-α supplemented with 10 mM β-glycerophosphate and 50 µg/mL magnesium ascorbyl phosphate. Statistical analysis was performed using ordinary one-way ANOVA followed by Dunnett’s tests versus the OP (or the OM) only group at the significance level of 0.05. 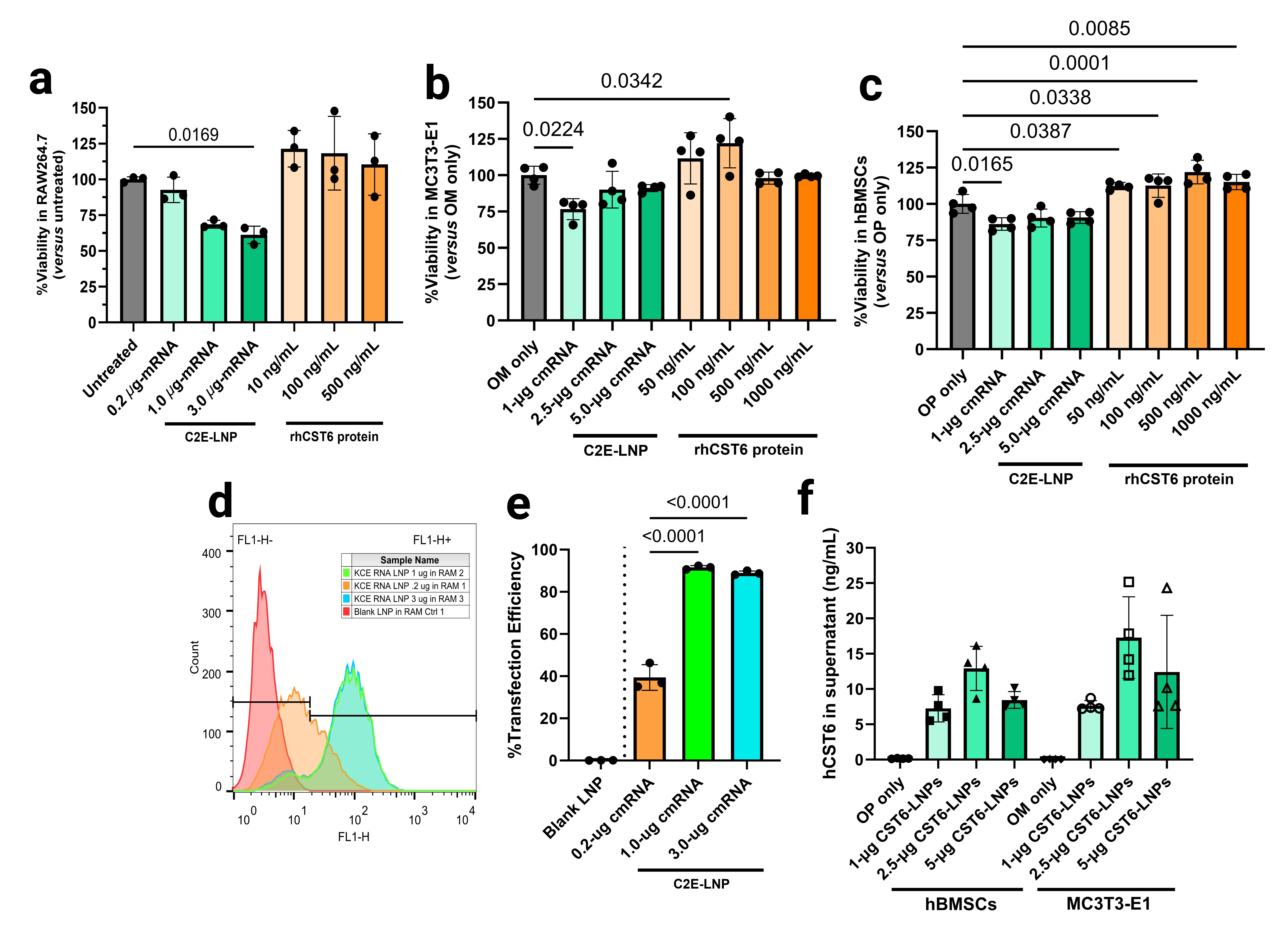 Figure 2: Cell viability, %transfection, and secreted hCST6 levels at 24 hours after hCST6-P2A-eGFP-coding cmRNA delivery (as lipid nanoparticles) in various cell types. MTS assays show minimal cytotoxicity in (a) RAW264.7 cells, (b) MC3T3-E1 subclone 4, and (c) hBMSCs. (d) & (e) Flow cytometry results show dose-dependent increases in transfection efficiencies. (f) ELISA results showing that CST6-LNPs delivery successfully induced expression and secretion of hCST6 protein in hBMSCs and MC3T3-E1 subclone 4 cells. OP = osteo-permissive medium; OM = osteogenic medium. OP and OM were of the same composition, which was MEM-α supplemented with 10% fetal bovine serum, 100 U/mL PenStrep, and 4 mM GlutaMAX™ with 10 mM β-glycerophosphate and 50 µg/mL magnesium ascorbyl phosphate. For (a), (b), and (c), statistical analysis was performed using ordinary one-way ANOVA followed by Dunnett’s tests versus the medium only group (i.e., untreated, OM only, or OP only) at the significance level of 0.05. For (e) and (f), statistical analysis was performed using ordinary one-way ANOVA followed by Tukey’s tests (with exclusion of background control groups, namely Blank LNP, OP only, and OM only) at the significance level of 0.05.
Figure 2: Cell viability, %transfection, and secreted hCST6 levels at 24 hours after hCST6-P2A-eGFP-coding cmRNA delivery (as lipid nanoparticles) in various cell types. MTS assays show minimal cytotoxicity in (a) RAW264.7 cells, (b) MC3T3-E1 subclone 4, and (c) hBMSCs. (d) & (e) Flow cytometry results show dose-dependent increases in transfection efficiencies. (f) ELISA results showing that CST6-LNPs delivery successfully induced expression and secretion of hCST6 protein in hBMSCs and MC3T3-E1 subclone 4 cells. OP = osteo-permissive medium; OM = osteogenic medium. OP and OM were of the same composition, which was MEM-α supplemented with 10% fetal bovine serum, 100 U/mL PenStrep, and 4 mM GlutaMAX™ with 10 mM β-glycerophosphate and 50 µg/mL magnesium ascorbyl phosphate. For (a), (b), and (c), statistical analysis was performed using ordinary one-way ANOVA followed by Dunnett’s tests versus the medium only group (i.e., untreated, OM only, or OP only) at the significance level of 0.05. For (e) and (f), statistical analysis was performed using ordinary one-way ANOVA followed by Tukey’s tests (with exclusion of background control groups, namely Blank LNP, OP only, and OM only) at the significance level of 0.05. 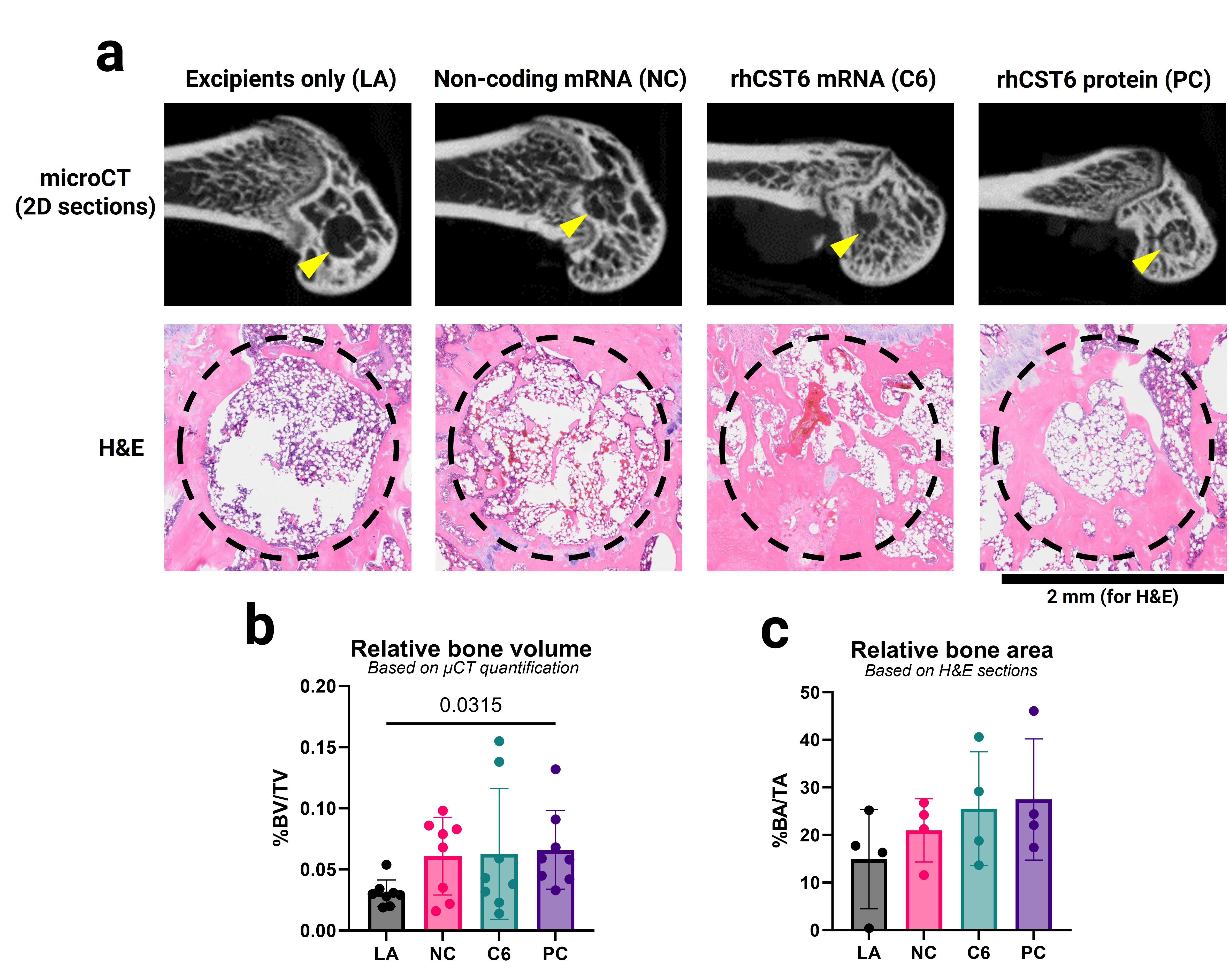 Figure 3: In vivo bone regeneration in the rat femoral metaphysis drill defect model. (a) representative 2D radiographs and H&E sections from each treatment group. Relative bone volumes and relative bone areas were quantified based on (b) micro-CT image reconstruction (n = 8 per treatment group) and based on (c) H&E sections (using ImageJ; n = 4 per treatment group). LA = collagen plug lyophilized with the buffer/cryoprotectant mix only (8%w/v sucrose in 5-mM Tris-HCl, pH 8); NC = collagen plug lyophilized with LNPs loaded with non-coding cmRNA in the buffer/cryoprotectant mix (CST6 sequence with scrambled Kozak sequence and in-frame stop codons); C6 = collagen plug lyophilized with LNPs loaded with CST6-coding cmRNA in the buffer/cryoprotectant mix; and PC = rhCST6 protein lyophilized in PBS, pH 7.4 (similar to the lyophilized product as supplied by the manufacturer of rhCST6 protein). NC and C6 were given as a 25 µg-RNA dose per defect; PC was given as a 15 µg-protein per defect; LA was loaded with the same volume of the buffer/cryoprotectant mix as NC and C6 prior to lyophilization. Collagen plug dimensions prior to lyophilization were 2.0 mm in diameter and 5.0 mm in length. The drill defect dimensions prior to implantation were 2.0 mm in diameter and 4.0 mm in depth. Statistical analysis was performed using ordinary one-way ANOVA followed by Dunnett’s tests versus the LA group at the significance level of 0.05.
Figure 3: In vivo bone regeneration in the rat femoral metaphysis drill defect model. (a) representative 2D radiographs and H&E sections from each treatment group. Relative bone volumes and relative bone areas were quantified based on (b) micro-CT image reconstruction (n = 8 per treatment group) and based on (c) H&E sections (using ImageJ; n = 4 per treatment group). LA = collagen plug lyophilized with the buffer/cryoprotectant mix only (8%w/v sucrose in 5-mM Tris-HCl, pH 8); NC = collagen plug lyophilized with LNPs loaded with non-coding cmRNA in the buffer/cryoprotectant mix (CST6 sequence with scrambled Kozak sequence and in-frame stop codons); C6 = collagen plug lyophilized with LNPs loaded with CST6-coding cmRNA in the buffer/cryoprotectant mix; and PC = rhCST6 protein lyophilized in PBS, pH 7.4 (similar to the lyophilized product as supplied by the manufacturer of rhCST6 protein). NC and C6 were given as a 25 µg-RNA dose per defect; PC was given as a 15 µg-protein per defect; LA was loaded with the same volume of the buffer/cryoprotectant mix as NC and C6 prior to lyophilization. Collagen plug dimensions prior to lyophilization were 2.0 mm in diameter and 5.0 mm in length. The drill defect dimensions prior to implantation were 2.0 mm in diameter and 4.0 mm in depth. Statistical analysis was performed using ordinary one-way ANOVA followed by Dunnett’s tests versus the LA group at the significance level of 0.05. 