Formulation and Delivery - Biomolecular
(M1330-08-49) Aggressive But Controlled: Pushing the Limits of mAb Freeze-Drying in an 8-Hour Cycle

Hesham Refaat, MS (he/him/his)
Graduate Research Assistant, College of Pharmacy, Department of PSET
University of Iowa
Iowa City, Iowa, United States
Hesham Refaat, MS (he/him/his)
Graduate Research Assistant, College of Pharmacy, Department of PSET
University of Iowa
Iowa City, Iowa, United States- HG
Hima Bindu Gottam, MS, MBA, RAC (US) (she/her/hers)
Formulation Director
University of Iowa
Iowa City, Iowa, United States - AA
Aziz Ahmad, Ph.D.
Postdoc
University of Minnesota
Minnapolis, Minnesota, United States - JP
John Patani, MS
Graduate Research Assistant
University of Minnesota
Minnapolis, Minnesota, United States 
Tze Ning Hiew, PhD
Assistant Professor
University of Iowa
Iowa City, Iowa, United States
Vadim J. Gurvich, PhD
Research Associate Professor of Medicinal Chemistry
University of Minnesota
Minneapolis, Minnesota, United States
Raj Suryanarayanan, Ph.D.
Professor of Pharmaceutics
University of Minnesota
Minnapolis, Minnesota, United States- RN
Reza Nejadnik, PhD
Associate Professor
University of Iowa
Iowa City, Iowa, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: Five monoclonal antibody formulations were prepared using sucrose (F1, 1:1 w/w protein-to-sugar), sorbitol (F2, 1:1), and trehalose at three concentrations: F3 (1:1), F4 (1:2), and F5 (1:3). All were subjected to an aggressive freeze-drying cycle totaling 8 hours, performed at a constant chamber pressure of 300 mTorr. Freezing involved a two-step ramp to -45 °C. The primary drying phase began at –20 °C and ramped to 0 °C while reducing chamber pressure. Secondary drying was conducted in four rapid shelf temperature steps from 10 °C to 30 °C, followed by a 60-minute final hold. Hold times were minimized to compress the overall cycle without compromising drying endpoints. Post-lyophilization assessments included cake morphology, reconstitution time, and aggregation (size exclusion chromatography).
Results: All five formulations (F1–F5) were successfully lyophilized using the aggressive 8-hour cycle. F1 (sucrose) and F2 (sorbitol) had no cake cracking and structural defects, while F3 (trehalose 1:1), F4 (trehalose 1:2), and F5 (trehalose 1:3) exhibited cracking in 3, 2, and 1 vials, respectively. Reconstitution time was shortest in F2 (33.75 ± 2.95 s) and longest in F4 (82.33 ± 11.85 s), with trehalose-based formulations (F3–F5) requiring longer reconstitution than F1 and F2. High molecular weight species (HMW%) remained consistent across formulations (0.429–0.480%), indicating no significant aggregation under the accelerated process (table 1). All formulations produced visually acceptable cakes with intact structure (figure 1). Placebo formulations containing trehalose formed cohesive cakes even without protein, unlike those with sucrose or sorbitol, and increasing trehalose concentration led to more opaque and well-defined cake structures (figure 2).
Conclusion: This study demonstrates that an aggressive 8-hour lyophilization cycle could be applied to the studied monoclonal antibody formulations without compromising stability. All formulations retained acceptable levels of high molecular weight species post-lyophilization. Trehalose-based formulations formed cohesive and robust cakes even in the absence of protein which shows trehalose’s ability to support matrix structure independently. Increasing trehalose concentration led to more opaque and denser cakes. In contrast, sucrose and sorbitol resulted in weaker matrix structures with faster reconstitution. While these findings support the feasibility of aggressive cycle compression in biologics manufacturing, caution should be exercised when extrapolating to other proteins and process conditions, as the monoclonal antibody used here may inherently possess high stability under the applied stress.
References: Gao et al, "Effect of annealing on visible-bubble formation and stability profiles of freeze-dried high concentration omalizumab formulations." Molecular Pharmaceutics 21, no. 4 (2024): 1691-1704.
Najarian et al, "Optimizing lyophilization primary drying: A vaccine case study with experimental and modeling techniques." International Journal of Pharmaceutics 659 (2024): 124168
Acknowledgements: This project is supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of a financial assistance award to the National Institute for Pharmaceutical Technology and Education (NIPTE). The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by FDA/HHS, or the U.S. Government.
.jpg) Table 1. Summary of formulation composition, reconstitution time, and high molecular weight species (HMW% ) following aggressive lyophilization cycle.
Table 1. Summary of formulation composition, reconstitution time, and high molecular weight species (HMW% ) following aggressive lyophilization cycle..jpg) Figure 1. Side-view images of lyophilized cakes for formulations F1–F5, captured through the vial wall under light microscopy
Figure 1. Side-view images of lyophilized cakes for formulations F1–F5, captured through the vial wall under light microscopy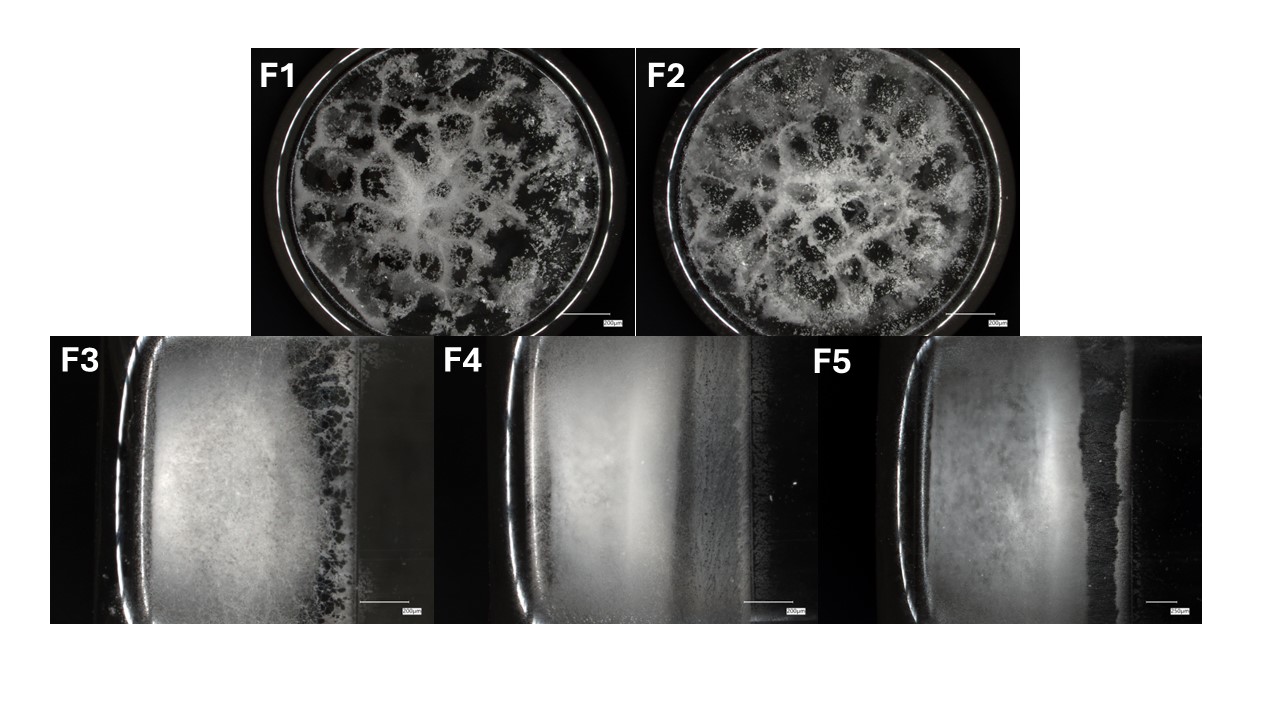 Figure 2. Bottom- and side-view images of lyophilized placebo formulations (F1–F5), captured through the vial wall or top surface. Increased trehalose concentration correlated with greater opacity and improved structural definition while sucrose (F1) and sorbitol (F2) yielded more fragmented or collapsed matrices.
Figure 2. Bottom- and side-view images of lyophilized placebo formulations (F1–F5), captured through the vial wall or top surface. Increased trehalose concentration correlated with greater opacity and improved structural definition while sucrose (F1) and sorbitol (F2) yielded more fragmented or collapsed matrices.