Formulation and Delivery - Chemical
(M1530-09-61) Development and Optimization of Liquisolid Compacts of Febuxostat Using Conventional Processing and Twin-Screw Granulation: A Comparative Study for Solubility and Dissolution Enhancement

Prateek Uttreja, MS
PhD Student
University of Mississippi
Oxford, Mississippi, United States
Prateek Uttreja, MS
PhD Student
University of Mississippi
Oxford, Mississippi, United States- IK
Indrajeet Karnik, MS
Graduate Student
University of Mississippi
University, Mississippi, United States - NN
Nagarjuna Narala, MS (he/him/his)
PhD Student
University of Mississippi
Oxford, Mississippi, United States - SB
Srikanth Baisa, MS
PhD Student
University of Mississippi
Oxford, Mississippi, United States 
Rasha M. Elkanayati, MS
Graduate Research Assistant
University of Mississippi
Oxford, Mississippi, United States- NA
Nouf Alshammari, MS (she/her/hers)
Graduate Student
University of Mississippi
Oxford, Mississippi, United States - EA
Esraa Al Shawakri, Ph.D.
PhD Student
University of Mississippi
Oxford, Mississippi, United States - SV
Sateesh Kumar Vemula, Ph.D. (he/him/his)
Post-doc
University of Mississippi
Oxford, Mississippi, United States - MA
Michael A. A. Repka, Ph.D.
DISTINGUISHED PROFESSOR PHARMACEUTICS & DRUG DELIVERY
University of Mississippi
Oxford, Mississippi, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: Saturation solubility screening was initially performed to identify a suitable liquid vehicle. Transcutol HP showed the highest solubility for Febuxostat and was selected for further formulation. Mortar-pestle-based liquisolid compacts and twin-screw granulated formulations were both developed using a fixed drug concentration (10% w/w), varying the concentrations of Transcutol HP, lactose, mannitol, and Aerosil 200, along with 5% croscarmellose sodium as a disintegrant. Solubility studies were conducted in pH 1.2, pH 4.5, and water to compare the solubility of pure drug, mortar-pestle compacts, and twin-screw granules. PXRD and DSC were used to evaluate the crystalline-amorphous transitions and molecular state of the drug. SEM was performed to assess surface morphology. Dissolution studies were conducted using USP Apparatus II at 75 rpm in pH 1.2, pH 4.5, and water, comparing the pure drug, conventional formulation, and the twin-screw granulated optimized formulation. A Box-Behnken design with two independent variables—percentage of liquid vehicle Transcutol HP (X1, 10–25%) and ratio of carriers (lactose/mannitol): Coating material (Aerosil 200) (X2, 5:1 to 15:1) was employed to study their effects on percent drug release at 60 min (Y1) and disintegration time (Y2).
Results: The screening study revealed Transcutol HP to be the most effective liquid vehicle for dissolving Febuxostat. The saturation solubility of the pure drug in pH 1.2 was 4.3 µg/mL, in pH 4.5 it was 28.7 µg/mL, and in water it was 12.5 µg/mL. The optimized mortar-pestle-based formulation showed increased solubility: 12.8 µg/mL (pH 1.2), 68.4 µg/mL (pH 4.5), and 49.3 µg/mL (water), while the Twin Screw Granulation-based optimized formulation further enhanced solubility to 18.9 µg/mL, 85.6 µg/mL, and 62.7 µg/mL in respective media. In vitro dissolution data indicated a significant enhancement in release from both formulations compared to the pure drug. After 60 minutes in water, the pure drug showed 6.5% release, the mortar-pestle formulation (F4) showed 34.1%, and the twin-screw granulated counterpart showed 46.2%. At pH 1.2, the pure drug released only 0.3%, whereas the conventional and twin-screw formulations released 0.9% and 6.0%, respectively. At pH 4.5, drug release improved to 3.6% (pure drug), 14.3% (conventional), and 18.6% (TSG). PXRD and DSC confirmed partial amorphization of febuxostat in liquisolid compacts, more prominent in TSG formulations. SEM images demonstrated improved surface smoothness and drug dispersion in TSG granules. A Box–Behnken Design identified the optimized formulation containing 15% Transcutol HP and a carrier (lactose:mannitol 1:1) coating (Aerosil 200) ratio of 14:1, achieving 46.2% drug release in water at 60 minutes and disintegration time below 6 minutes.
Conclusion: The study demonstrated that the twin-screw granulation-based liquisolid compacts provided superior enhancement in solubility and dissolution of Febuxostat compared to conventional mortar-pestle mixing. Transcutol HP was established as the most suitable liquid vehicle based on preliminary screening. Enhanced solubility in biorelevant media and improved drug release from twin-screw formulations indicate the potential of this continuous processing method for industrial scalability. Box-Behnken optimization effectively defined the critical formulation parameters and their ideal levels. The findings support the utility of twin-screw granulation as a robust alternative to conventional mixing in liquisolid technology for poorly water-soluble drugs.
References: Mei Lu, Haonan Xing, Jingzheng Jiang, Xiao Chen, Tianzhi Yang, Dongkai Wang, Pingtian Ding, Liquisolid technique and its applications in pharmaceutics, Asian Journal of Pharmaceutical Sciences, Volume 12, Issue 2, 2017,Pages 115-123, ISSN 1818-0876, https://doi.org/10.1016/j.ajps.2016.09.007.
.jpg) Figure 1: Scanning Electron Microscopy (SEM) images of (A) pure febuxostat crystals showing rod-shaped crystalline morphology, (B) liquisolid compact prepared by the conventional blending method demonstrating surface embedding of drug within the carrier matrix, and (C) liquisolid granules prepared via twin screw granulation revealing uniform, agglomerated granules with smoother morphology and enhanced surface dispersion.
Figure 1: Scanning Electron Microscopy (SEM) images of (A) pure febuxostat crystals showing rod-shaped crystalline morphology, (B) liquisolid compact prepared by the conventional blending method demonstrating surface embedding of drug within the carrier matrix, and (C) liquisolid granules prepared via twin screw granulation revealing uniform, agglomerated granules with smoother morphology and enhanced surface dispersion.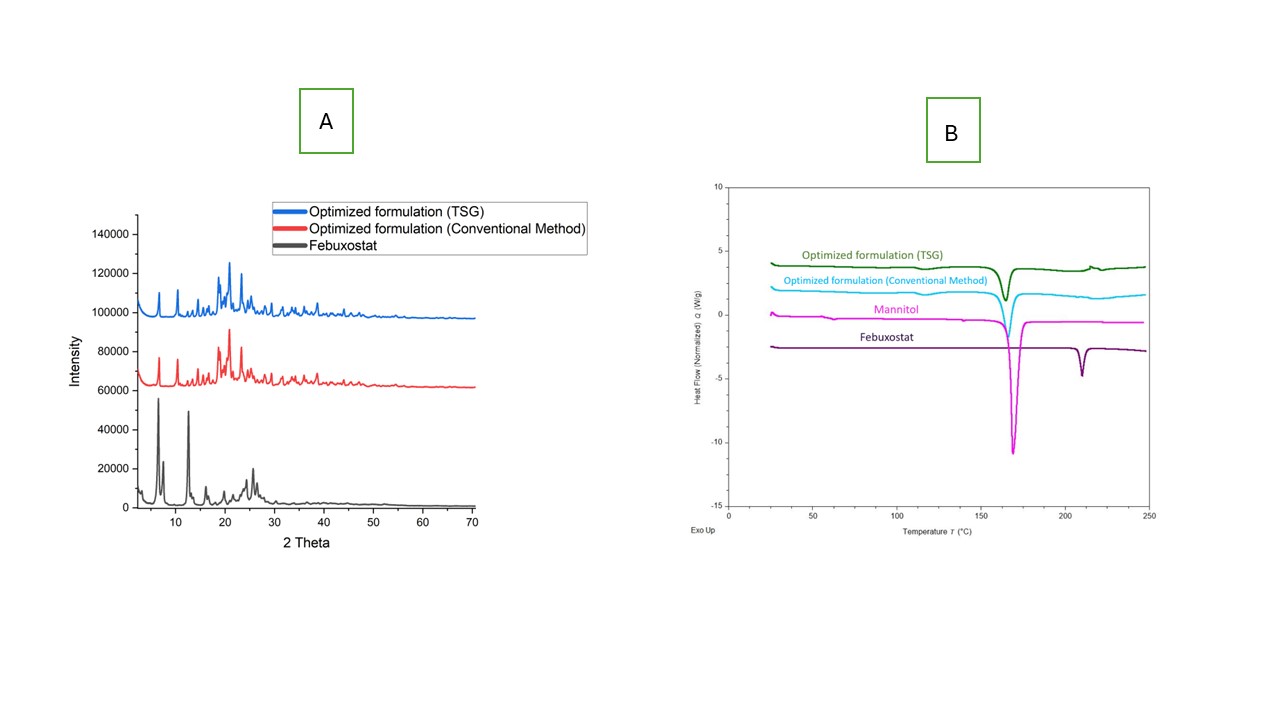 Figure 2: (A) Powder X-ray Diffraction (PXRD) patterns of pure Febuxostat, optimized formulation prepared via conventional blending, and Twin Screw Granulation (TSG) method. The sharp crystalline peaks of pure febuxostat diminished significantly in both formulations, indicating partial amorphization, with greater reduction observed in the TSG-based system. (B) Differential Scanning Calorimetry (DSC) thermograms of pure Febuxostat, Mannitol, and optimized formulations. The characteristic melting endotherm of febuxostat (~205°C) was reduced or absent in the formulations, confirming reduced crystallinity and potential molecular dispersion within the excipient matrix.
Figure 2: (A) Powder X-ray Diffraction (PXRD) patterns of pure Febuxostat, optimized formulation prepared via conventional blending, and Twin Screw Granulation (TSG) method. The sharp crystalline peaks of pure febuxostat diminished significantly in both formulations, indicating partial amorphization, with greater reduction observed in the TSG-based system. (B) Differential Scanning Calorimetry (DSC) thermograms of pure Febuxostat, Mannitol, and optimized formulations. The characteristic melting endotherm of febuxostat (~205°C) was reduced or absent in the formulations, confirming reduced crystallinity and potential molecular dispersion within the excipient matrix.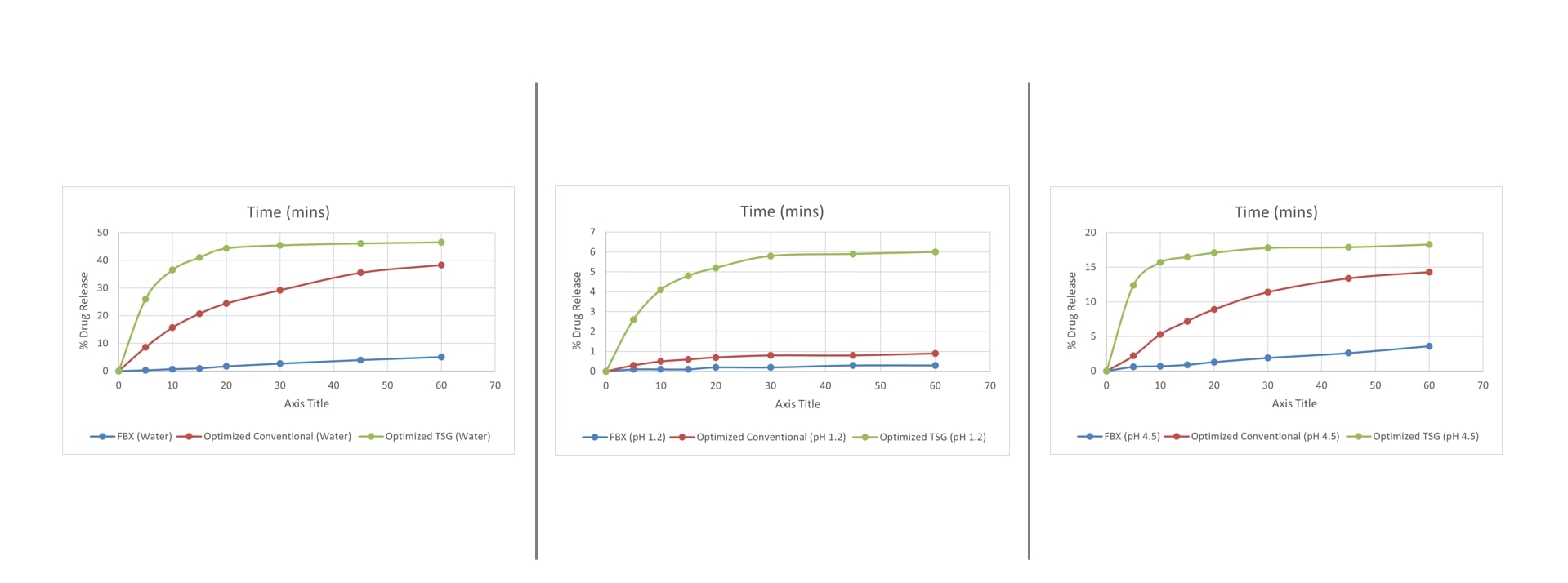 Figure 3: In vitro dissolution profiles of febuxostat, optimized conventional formulation, and optimized twin screw granulation (TSG) formulation in different media.
Figure 3: In vitro dissolution profiles of febuxostat, optimized conventional formulation, and optimized twin screw granulation (TSG) formulation in different media.