Preclinical, Clinical, and Translational Sciences
(T0930-05-29) Transdermal Delivery of Enfuvirtide Using Dissolving Microneedles Integrated with Insertion and Removal Indicator
Tuesday, November 11, 2025
9:30 AM - 10:30 AM CT

Huanhuan Li, Ph.D.
Research Fellow
Queen's University Belfast
BELFAST, Northern Ireland, United Kingdom
Huanhuan Li, Ph.D.
Research Fellow
Queen's University Belfast
BELFAST, Northern Ireland, United Kingdom
Ryan Donnelly, Ph.D. (he/him/his)
Professor
Queen's University Belfast
Belfast, Northern Ireland, United Kingdom
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Enfuvirtide, the first HIV fusion inhibitor, exhibits remarkable antiviral efficacy and a favorable safety profile compared with antiretroviral therapy alone. Nevertheless, its clinical application is constrained by the necessity for subcutaneous administration and the high incidence of injection site reactions (ISRs) in 98% of patients[1]. This study seeks to overcome these limitations by developing dissolving microneedle array patches (DMAPs), offering a painless and efficient alternative for enfuvirtide delivery.
Methods: Two bilayer DMAP designs featuring poly(vinylpyrrolidone) (PVP) based-hydrophilic and poly(lactic acid) (PLA)-based hydrophobic baseplates were engineered, incorporating a novel PLA-silica baseplate as a colorimetric dissolution indicator for visual feedback on successful patch insertion and timely removal. Comprehensive evaluations encompassing physicochemical attributes and biocompatibility of the systems, microneedle integrity and mechanical strength were conducted, alongside ex vivo dissolution and permeation studies of enfuvirtide. In vivo studies on rats were undertaken to assess the practical viability of this approach for the delivery of this peptide.
Results: The final optimised DMAPs demonstrated well-defined geometries, consistent microneedle heights, and precisely controlled interspacing, attributed to the uniform micro-mould design. These features enabled effective insertion into Parafilm®M models, with height reduction upon compression remaining below 20% (Figure 1). In vitro permeation and dissolution studies confirmed complete microneedle dissolution within 1 hour. Ex vivo antiviral assays against MT4 cells demonstrated the biocompatibility of the formulations and preserved anti-HIV activity of enfuvirtide following DMAP fabrication. The IC50 values were approximately 4.5 ng/mL for both the free drug and enfuvirtide incorporated into PVP-based DMAPs, while enfuvirtide delivered via PLA-based DMAPs exhibited a modest increase in IC50 (Figure 2). The DMAPs integrated with visual feedback indicators showed a rapid colour transition from yellow to green within 3 minutes post-insertion on rat skin triggered by microneedle hydration from interstitial fluid. This was followed by a shift to purple within 2 hours, indicating complete microneedle dissolution and confirming successful drug delivery. In vivo pharmacokinetic studies in Sprague Dawley rats revealed that the PLA-silica DMAP achieved a peak plasma concentration (Cmax) of 1864 ± 480 ng/mL at 0.5 hours, while the PVP-baseplate DMAP reached a Cmax of 973 ± 200 ng/mL at 1 hour. Both systems demonstrated good biocompatibility with no signs of irritation or adverse reactions (Figure 3).
Conclusion: These findings underscore DMAPs as a promising alternative to subcutaneous injection of enfuvirtide, capable of reducing ISRs and potentially enhancing patient adherence. Notably, this work introduced an innovative solution for prompt patch removal upon complete drug delivery, effectively addressing dosing inconsistencies and enabling individualised administration, which is crucial for ensuring the reliability and patient acceptability for widespread adoption of this technology.
References: [1] Jamjian, M C., McNicholl, I R., 2004. Enfuvirtide: first fusion inhibitor for treatment of HIV infection. American journal of health-system pharmacy, 61(12): 1242-1247.
[2]Li, H., Anjani, Q. K., Hutton, A. R., Paris, J. L., Moreno‐Castellanos, N., Himawan, A., ... & Donnelly, R. F. (2024). Design of a Novel Delivery Efficiency Feedback System for Biphasic Dissolving Microarray Patches Based on Poly (Lactic Acid) and Moisture‐Indicating Silica. Advanced Healthcare Materials, 13(17), 2304082.
Acknowledgements: This work was supported by China Scholarship Council (202106370014) and EPSRC grant EP/V047221/1.
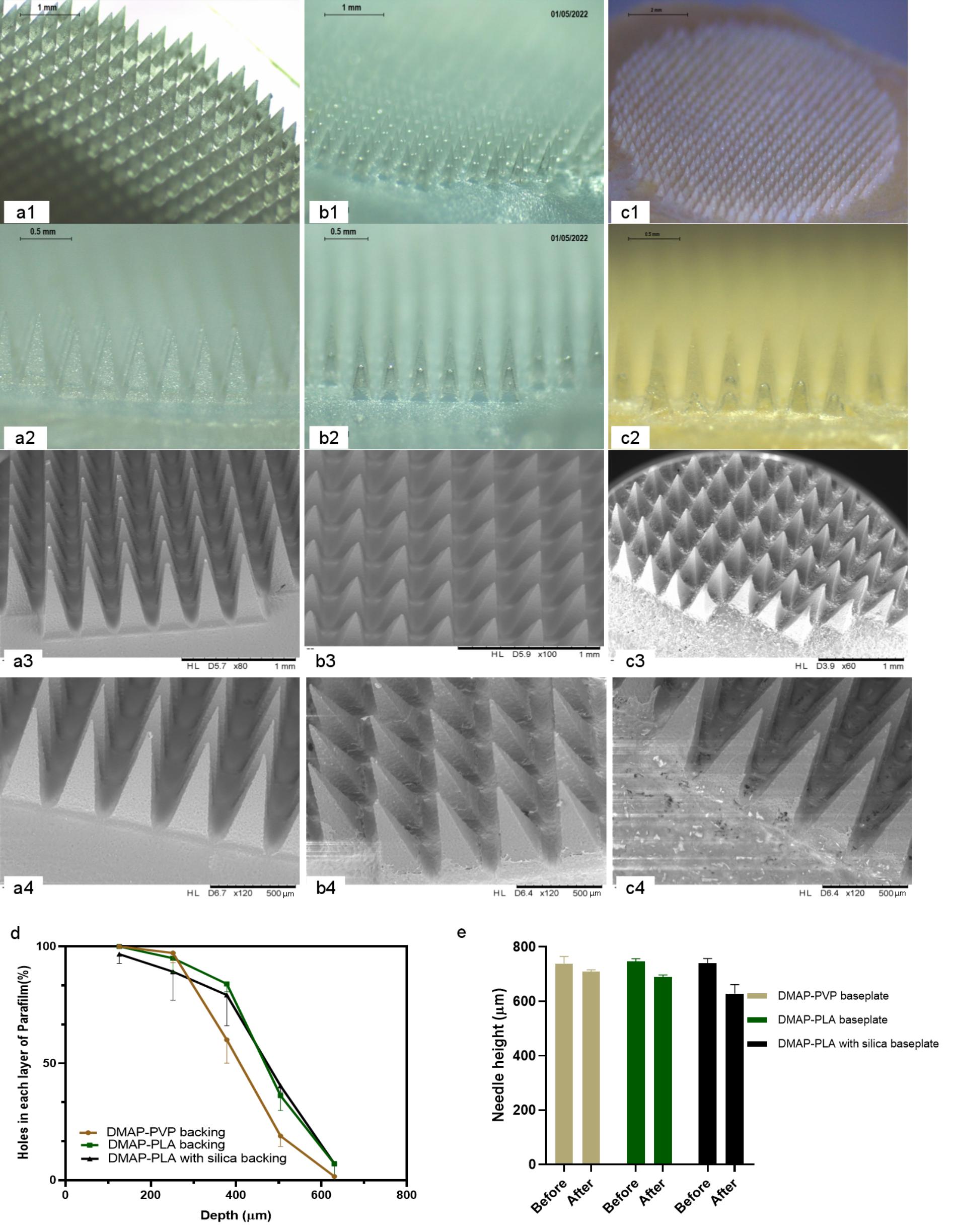 Figure 1. Morphology of PVP-DMAPs (a1-4), PLA-DMAPs (b1-4) and PLA silica-DMAPs (c1-4) obtained by optical microscope and SEM. Results of the Parafilm®M insertion test (d) (Means-SDs, n=5) and height reduction test (Means + SDs, n=5) (e) of DMAPs with PVP, PLA and PLA with a silica infued-baseplate.
Figure 1. Morphology of PVP-DMAPs (a1-4), PLA-DMAPs (b1-4) and PLA silica-DMAPs (c1-4) obtained by optical microscope and SEM. Results of the Parafilm®M insertion test (d) (Means-SDs, n=5) and height reduction test (Means + SDs, n=5) (e) of DMAPs with PVP, PLA and PLA with a silica infued-baseplate.
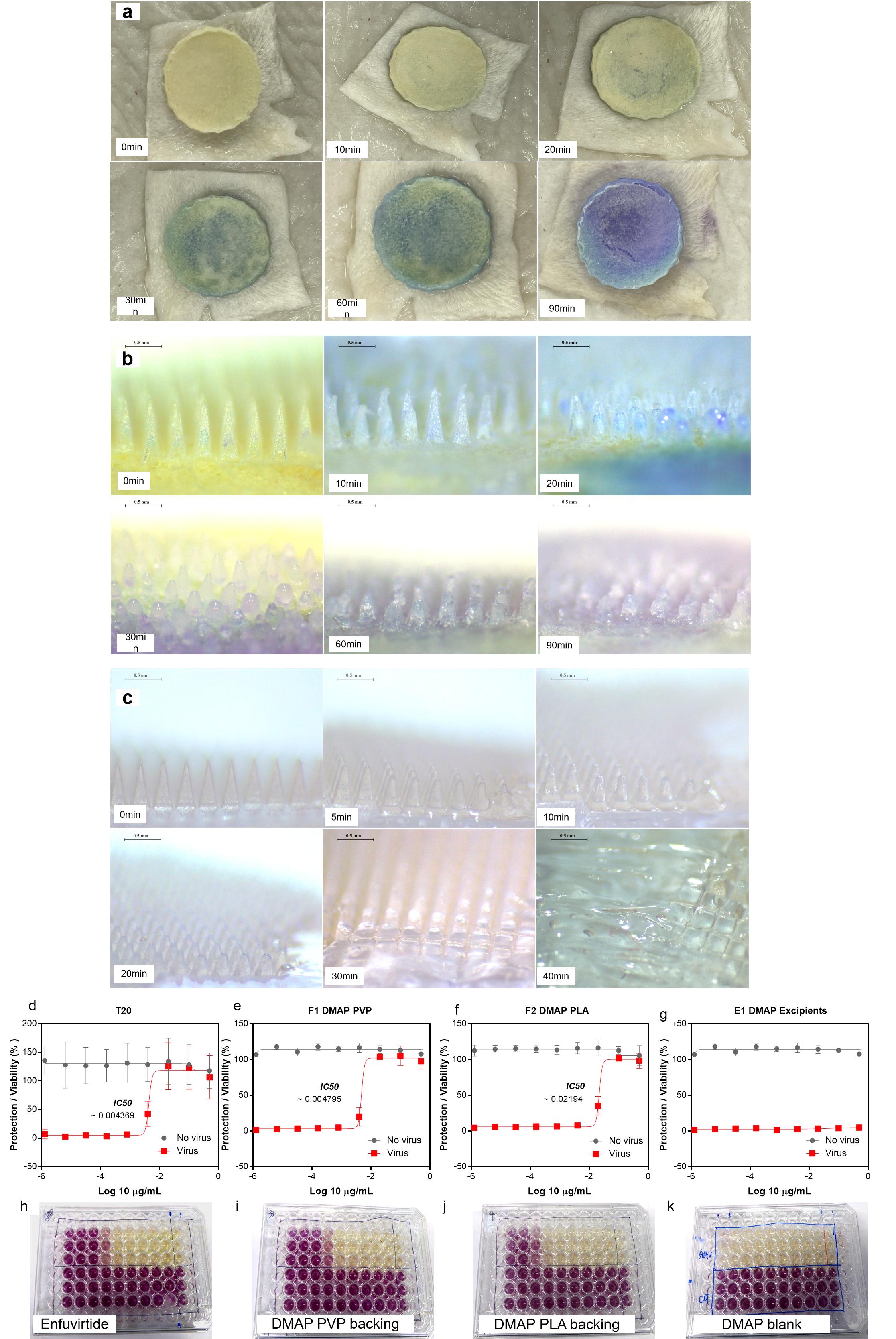 Figure 2. Schematic illustration of the fabrication process of DMAPs (a0). Morphology of dissolving microneedles of FITC-dextran (a-f) and fluorescein sodium (g-l) before (a-c&g-i) and after (d-f&j-l) needle dissolution obtained by optical microscopy and SEM. Representative fluorescence images and Z-stack images of DMAP of FITC-dextran 150 kDa (m, n, o) and fluorescein sodium (p, q, r) obtained by fluorescence microscope and multiphoton microscopy. The percentage of viable cells after 72 h of culture with PLA+M180 and PLA+M50 DMAPs in MTT assay (Means + SD, n=6) (k). Alive/dead staining of fibroblastic cells on control, PLA+M180 and PLA+M50 treated samples (l) (Green represented alive and red represented dead). Total DNA content of cells on control, PLA+M180 and PLA+M50 cultured for 72 h in PicoGreen assay (Means + SD, n=6) (m). ** represents p<0.001.
Figure 2. Schematic illustration of the fabrication process of DMAPs (a0). Morphology of dissolving microneedles of FITC-dextran (a-f) and fluorescein sodium (g-l) before (a-c&g-i) and after (d-f&j-l) needle dissolution obtained by optical microscopy and SEM. Representative fluorescence images and Z-stack images of DMAP of FITC-dextran 150 kDa (m, n, o) and fluorescein sodium (p, q, r) obtained by fluorescence microscope and multiphoton microscopy. The percentage of viable cells after 72 h of culture with PLA+M180 and PLA+M50 DMAPs in MTT assay (Means + SD, n=6) (k). Alive/dead staining of fibroblastic cells on control, PLA+M180 and PLA+M50 treated samples (l) (Green represented alive and red represented dead). Total DNA content of cells on control, PLA+M180 and PLA+M50 cultured for 72 h in PicoGreen assay (Means + SD, n=6) (m). ** represents p<0.001.
 Figure 3. Ex vivo dissolution (a, c) and baseplate colour change (b,d) for FITC-dextran (a, b) and fluorescein sodium (c, d) dissolving microneedle. Ex vivo delivery of the two dissolving microneedles (e) and distribution of the analytes (mean±SD, n = 3) (f). Preliminary result from dissolving microneedles with the feedback system on rats (g).
Figure 3. Ex vivo dissolution (a, c) and baseplate colour change (b,d) for FITC-dextran (a, b) and fluorescein sodium (c, d) dissolving microneedle. Ex vivo delivery of the two dissolving microneedles (e) and distribution of the analytes (mean±SD, n = 3) (f). Preliminary result from dissolving microneedles with the feedback system on rats (g).
Methods: Two bilayer DMAP designs featuring poly(vinylpyrrolidone) (PVP) based-hydrophilic and poly(lactic acid) (PLA)-based hydrophobic baseplates were engineered, incorporating a novel PLA-silica baseplate as a colorimetric dissolution indicator for visual feedback on successful patch insertion and timely removal. Comprehensive evaluations encompassing physicochemical attributes and biocompatibility of the systems, microneedle integrity and mechanical strength were conducted, alongside ex vivo dissolution and permeation studies of enfuvirtide. In vivo studies on rats were undertaken to assess the practical viability of this approach for the delivery of this peptide.
Results: The final optimised DMAPs demonstrated well-defined geometries, consistent microneedle heights, and precisely controlled interspacing, attributed to the uniform micro-mould design. These features enabled effective insertion into Parafilm®M models, with height reduction upon compression remaining below 20% (Figure 1). In vitro permeation and dissolution studies confirmed complete microneedle dissolution within 1 hour. Ex vivo antiviral assays against MT4 cells demonstrated the biocompatibility of the formulations and preserved anti-HIV activity of enfuvirtide following DMAP fabrication. The IC50 values were approximately 4.5 ng/mL for both the free drug and enfuvirtide incorporated into PVP-based DMAPs, while enfuvirtide delivered via PLA-based DMAPs exhibited a modest increase in IC50 (Figure 2). The DMAPs integrated with visual feedback indicators showed a rapid colour transition from yellow to green within 3 minutes post-insertion on rat skin triggered by microneedle hydration from interstitial fluid. This was followed by a shift to purple within 2 hours, indicating complete microneedle dissolution and confirming successful drug delivery. In vivo pharmacokinetic studies in Sprague Dawley rats revealed that the PLA-silica DMAP achieved a peak plasma concentration (Cmax) of 1864 ± 480 ng/mL at 0.5 hours, while the PVP-baseplate DMAP reached a Cmax of 973 ± 200 ng/mL at 1 hour. Both systems demonstrated good biocompatibility with no signs of irritation or adverse reactions (Figure 3).
Conclusion: These findings underscore DMAPs as a promising alternative to subcutaneous injection of enfuvirtide, capable of reducing ISRs and potentially enhancing patient adherence. Notably, this work introduced an innovative solution for prompt patch removal upon complete drug delivery, effectively addressing dosing inconsistencies and enabling individualised administration, which is crucial for ensuring the reliability and patient acceptability for widespread adoption of this technology.
References: [1] Jamjian, M C., McNicholl, I R., 2004. Enfuvirtide: first fusion inhibitor for treatment of HIV infection. American journal of health-system pharmacy, 61(12): 1242-1247.
[2]Li, H., Anjani, Q. K., Hutton, A. R., Paris, J. L., Moreno‐Castellanos, N., Himawan, A., ... & Donnelly, R. F. (2024). Design of a Novel Delivery Efficiency Feedback System for Biphasic Dissolving Microarray Patches Based on Poly (Lactic Acid) and Moisture‐Indicating Silica. Advanced Healthcare Materials, 13(17), 2304082.
Acknowledgements: This work was supported by China Scholarship Council (202106370014) and EPSRC grant EP/V047221/1.
 Figure 1. Morphology of PVP-DMAPs (a1-4), PLA-DMAPs (b1-4) and PLA silica-DMAPs (c1-4) obtained by optical microscope and SEM. Results of the Parafilm®M insertion test (d) (Means-SDs, n=5) and height reduction test (Means + SDs, n=5) (e) of DMAPs with PVP, PLA and PLA with a silica infued-baseplate.
Figure 1. Morphology of PVP-DMAPs (a1-4), PLA-DMAPs (b1-4) and PLA silica-DMAPs (c1-4) obtained by optical microscope and SEM. Results of the Parafilm®M insertion test (d) (Means-SDs, n=5) and height reduction test (Means + SDs, n=5) (e) of DMAPs with PVP, PLA and PLA with a silica infued-baseplate.  Figure 2. Schematic illustration of the fabrication process of DMAPs (a0). Morphology of dissolving microneedles of FITC-dextran (a-f) and fluorescein sodium (g-l) before (a-c&g-i) and after (d-f&j-l) needle dissolution obtained by optical microscopy and SEM. Representative fluorescence images and Z-stack images of DMAP of FITC-dextran 150 kDa (m, n, o) and fluorescein sodium (p, q, r) obtained by fluorescence microscope and multiphoton microscopy. The percentage of viable cells after 72 h of culture with PLA+M180 and PLA+M50 DMAPs in MTT assay (Means + SD, n=6) (k). Alive/dead staining of fibroblastic cells on control, PLA+M180 and PLA+M50 treated samples (l) (Green represented alive and red represented dead). Total DNA content of cells on control, PLA+M180 and PLA+M50 cultured for 72 h in PicoGreen assay (Means + SD, n=6) (m). ** represents p<0.001.
Figure 2. Schematic illustration of the fabrication process of DMAPs (a0). Morphology of dissolving microneedles of FITC-dextran (a-f) and fluorescein sodium (g-l) before (a-c&g-i) and after (d-f&j-l) needle dissolution obtained by optical microscopy and SEM. Representative fluorescence images and Z-stack images of DMAP of FITC-dextran 150 kDa (m, n, o) and fluorescein sodium (p, q, r) obtained by fluorescence microscope and multiphoton microscopy. The percentage of viable cells after 72 h of culture with PLA+M180 and PLA+M50 DMAPs in MTT assay (Means + SD, n=6) (k). Alive/dead staining of fibroblastic cells on control, PLA+M180 and PLA+M50 treated samples (l) (Green represented alive and red represented dead). Total DNA content of cells on control, PLA+M180 and PLA+M50 cultured for 72 h in PicoGreen assay (Means + SD, n=6) (m). ** represents p<0.001. Figure 3. Ex vivo dissolution (a, c) and baseplate colour change (b,d) for FITC-dextran (a, b) and fluorescein sodium (c, d) dissolving microneedle. Ex vivo delivery of the two dissolving microneedles (e) and distribution of the analytes (mean±SD, n = 3) (f). Preliminary result from dissolving microneedles with the feedback system on rats (g).
Figure 3. Ex vivo dissolution (a, c) and baseplate colour change (b,d) for FITC-dextran (a, b) and fluorescein sodium (c, d) dissolving microneedle. Ex vivo delivery of the two dissolving microneedles (e) and distribution of the analytes (mean±SD, n = 3) (f). Preliminary result from dissolving microneedles with the feedback system on rats (g).