Formulation and Delivery - Chemical
(T1130-09-61) Stability Evaluation of 4-Phenylbutyric Acid-Loaded Micro/Nano-Particulate Formulations for Topical Delivery Against Chemical Warfare Agent-Induced Skin Injury Using ICH Accelerated and Refrigerated Conditions
Tuesday, November 11, 2025
11:30 AM - 12:30 PM CT

Nethra Viswaroopan, BS
Ph.D. Student
Mercer University
Atlanta, Georgia, United States
Nethra Viswaroopan, BS
Ph.D. Student
Mercer University
Atlanta, Georgia, United States
Meheli Ghosh, MS
Graduate Student
Mercer University
Atlanta, Georgia, United States- JK
Jasim Khan, PhD
PhD
University of Alabama
Atlanta, Georgia, United States - RS
Ritesh K. Srivastava, Ph.D.
Post doc
University of Alabama
Birmingham, Alabama, United States - MA
Mohammad Athar, Ph.D.
Professor
University of Alabama
Atlanta, Georgia, United States 
Ajay K. K. Banga, PhD
Professor
Mercer University
Atlanta, Georgia, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Lewisite, a vesicating chemical warfare agent, represents a critical public health threat due to its ability to induce severe skin injuries, characterized by skin erythema and blistering. Effective response strategies require not only rapid decontamination but sustained topical therapeutic interventions to mitigate long-term tissue damage. 4-Phenylbutyric acid (4-PBA), a small molecule approved for the treatment of urea cycle disorders, has been identified as a potential therapeutic candidate due to its ability to attenuate endoplasmic reticulum stress and promote cellular recovery following arsenical exposure. To enhance 4-PBA's skin delivery profile, nanoparticle-based formulations were developed, including two distinct chitosan nanoparticle systems (N31 and N35), an emulsome (E2), and a microsponge (MS) system. The objective of the current study was to systematically evaluate the physicochemical, chemical, and thermal stability of these four nanoparticles under two accelerated storage conditions, as outlined in the ICH Q1A(R2) guidelines to ultimately identify a lead candidate for integration into a foam-based decontamination platform.
Methods: All nanoparticles were prepared using previously optimized protocols ensuring consistent size, drug loading, and morphology. Formulations were aliquoted into sealed containers and stored under two ICH stability conditions: refrigerated (25°C ± 2°C/60% RH ± 5%) and accelerated (40°C ± 2°C/75% RH ± 5%). Samples were withdrawn monthly for analysis. Particle size and polydispersity index (PDI) were measured by dynamic light scattering (DLS) using a Zetasizer Nano ZS. Zeta potential was measured to assess surface charge and colloidal stability. Drug content was quantified via high-performance liquid chromatography (HPLC) using a validated method for 4-PBA detection. Chemical stability was monitored by Fourier Transform Infrared (FT-IR) spectroscopy to identify peak shifts or disappearance suggestive of degradation or interaction. Differential Scanning Calorimetry (DSC) was used to assess thermal stability by monitoring the preservation of characteristic endothermic events. Visual observations were recorded at each timepoint to detect signs of aggregation, precipitation, or phase separation.
Results: Particle size measurements indicated that N31 remained the most stable among all formulations. At 40°C, N31 particle size increased slightly from 180.4 ± 4.2 nm at Month 0 to 187.6 ± 5.0 nm at Month 3. In comparison, N35 demonstrated significant instability at elevated temperature, with size increasing from 210.5 ± 6.1 nm to 320.8 ± 12.3 nm by Month 3. E2 exhibited moderate increases in size under accelerated conditions but remained relatively stable at 25°C. Microsponge (MS) formulations showed smaller size fluctuations, consistent with their robust porous structure. PDI values for N31 remained consistently below 0.35 across both conditions, indicating uniform size distribution. N35, however, displayed substantial PDI increases under accelerated conditions, rising from 0.38 ± 0.03 at Month 0 to 0.71 ± 0.05 at Month 3, reflecting particle aggregation and loss of uniformity. E2 PDI similarly increased from 0.31 to 0.62 at 40°C, whereas MS PDI values remained relatively moderate across both storage conditions. Zeta potential analysis further confirmed stability trends. N31 maintained a consistent zeta potential of -33.4 ± 2.1 mV, with minimal changes over time under both storage conditions. In contrast, E2 demonstrated a marked reduction in surface charge magnitude under accelerated storage, dropping from -47.8 ± 3.5 mV to -29.5 ± 3.2 mV, suggesting a loss of colloidal stability and a greater propensity for aggregation. MS formulations maintained zeta potentials between -20 and -30 mV, with minimal drift. Drug content analysis showed that N31 preserved over 90% of initial 4-PBA content at both 25°C and 40°C after 3 months. E2 retained between 85% and 88% of its original drug content. N35 demonstrated significant drug degradation at 40°C, retaining only ~65% of its original drug content by Month 3. Microsponge systems maintained approximately 80–85% drug content under both storage conditions. FT-IR spectra confirmed the chemical stability of N31, with no major peak shifts or loss of characteristic bands observed across all timepoints. In contrast, E2 and N35 exhibited minor band shifts and intensity changes near 1600–1700 cm⁻¹ under 40°C conditions, consistent with chemical degradation or molecular rearrangements. Microsponge formulations showed minimal spectral changes. DSC analysis revealed that N31 maintained sharp, distinct endothermic transitions even after 3 months at 40°C, confirming high thermal stability. E2 and N35 lost clear thermal events by Month 2–3 under accelerated conditions, indicating partial degradation and loss of crystalline structure. MS showed moderate thermal broadening but maintained identifiable transition peaks.
Conclusion: N31 chitosan nanoparticles demonstrated superior physicochemical, chemical, and thermal stability under both refrigerated and accelerated storage conditions compared to E2, N35, and MS formulations. N31 retained particle size uniformity, surface charge, drug content, chemical structure, and thermal properties across 3 months of testing. E2 and N35 exhibited significant size increases, PDI broadening, drug content loss, FT-IR peak shifts, and disappearance of thermal transitions under accelerated conditions, suggesting instability. Microsponge formulations showed intermediate stability. Overall, these findings support the selection of N31 as the lead candidate for foam-based decontamination and sustained delivery of 4-PBA for chemical exposure management.
References: 1)Kshirsagar, S.M., Viswaroopan, N., Ghosh, M. et al. Development of 4-phenylbutyric acid microsponge gel formulations for the treatment of lewisite-mediated skin injury. Drug Deliv. and Transl. Res. 15, 638–654 (2025). https://doi.org/10.1007/s13346-024-01620-y
2)Ghosh, M., Viswaroopan, N., Kshirsagar, S. M., Khan, J., Mohiuddin, S., Srivastava, R. K., Athar, M., & Banga, A. K. (2025). Sustained delivery of 4-phenylbutyric acid via chitosan nanoparticles in foam for decontamination and treatment of lewisite-mediated skin injury. International Journal of Pharmaceutics, 682, 125928. https://doi.org/10.1016/j.ijpharm.2025.125928
Acknowledgements: This project was funded by NIH/NIAMS U01AR078544
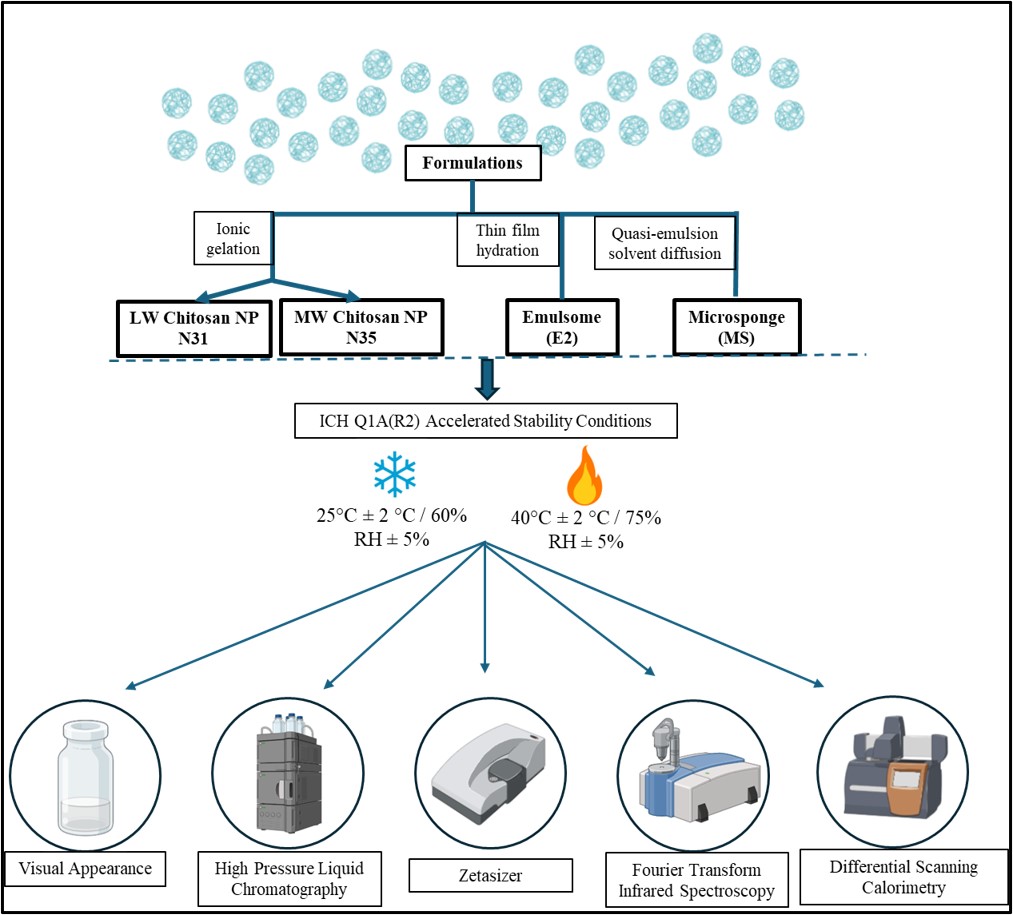 Fig 1. Schematic representation of the stability testing workflow for nanoparticle formulations
Fig 1. Schematic representation of the stability testing workflow for nanoparticle formulations
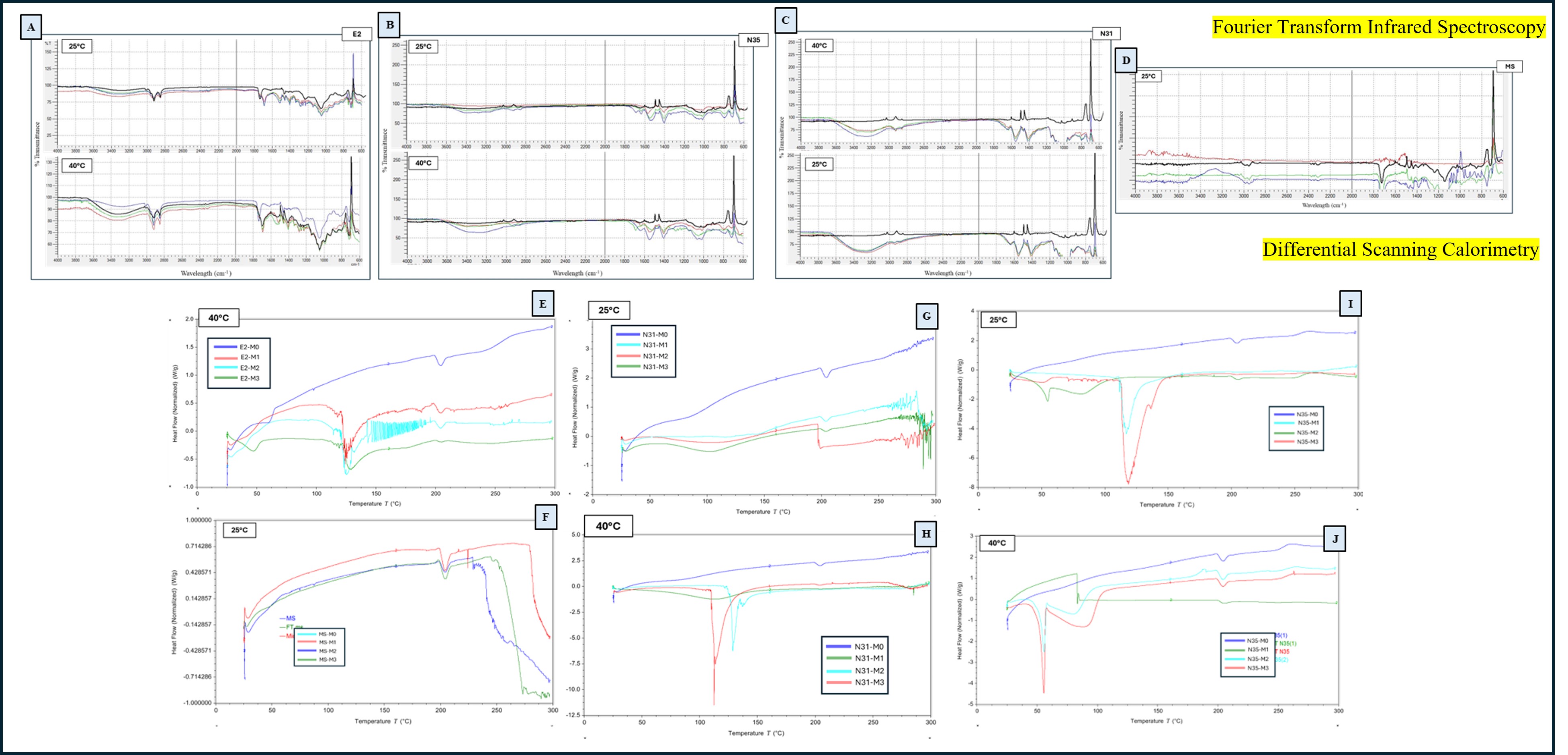 Fig 2. (A) FT-IR overlay of E2 nanoparticles stored at 25°C and 40°C over 3 months (B) FT-IR overlay of N35 nanoparticles stored at 25°C and 40°C (C) FT-IR overlay of N31 nanoparticles at 25°C and 40°C (D) FT-IR overlay of MS nanoparticles at 25°C . (E) DSC thermograms of E2 formulations at 40°C for 3 months (F) DSC thermograms of MS formulations at 25°C for 3 months (G) DSC thermograms of N31 nanoparticles at 25°C for 3 months (H) DSC thermograms of N31 nanoparticles at 40°C for 3 months (I) DSC thermograms of N35 nanoparticles at 25°C for 3 months (J) DSC thermograms of N35 nanoparticles at 40°C for 3 months.
Fig 2. (A) FT-IR overlay of E2 nanoparticles stored at 25°C and 40°C over 3 months (B) FT-IR overlay of N35 nanoparticles stored at 25°C and 40°C (C) FT-IR overlay of N31 nanoparticles at 25°C and 40°C (D) FT-IR overlay of MS nanoparticles at 25°C . (E) DSC thermograms of E2 formulations at 40°C for 3 months (F) DSC thermograms of MS formulations at 25°C for 3 months (G) DSC thermograms of N31 nanoparticles at 25°C for 3 months (H) DSC thermograms of N31 nanoparticles at 40°C for 3 months (I) DSC thermograms of N35 nanoparticles at 25°C for 3 months (J) DSC thermograms of N35 nanoparticles at 40°C for 3 months.
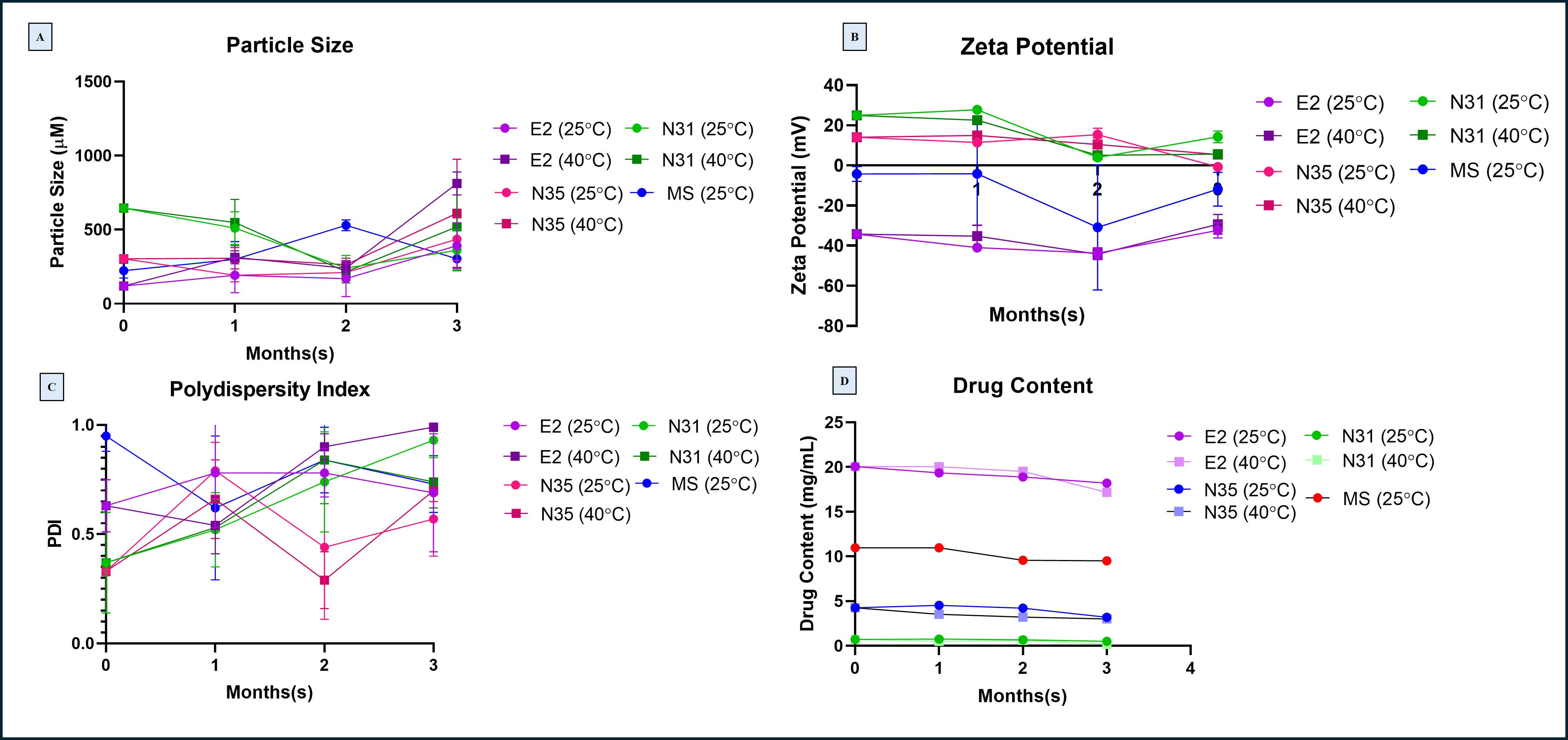 Fig 3. (A) Particle size of N31, N35, E2, and MS nanoparticles measured over 3 months at 25°C and 40°C storage conditions (B) Zeta potential values of nanoparticle formulations during stability study (C) Polydispersity index (PDI) of nanoparticles across 3 months, indicating changes in size distribution (D) Drug content of 4-PBA in nanoparticles measured monthly by HPLC
Fig 3. (A) Particle size of N31, N35, E2, and MS nanoparticles measured over 3 months at 25°C and 40°C storage conditions (B) Zeta potential values of nanoparticle formulations during stability study (C) Polydispersity index (PDI) of nanoparticles across 3 months, indicating changes in size distribution (D) Drug content of 4-PBA in nanoparticles measured monthly by HPLC
Methods: All nanoparticles were prepared using previously optimized protocols ensuring consistent size, drug loading, and morphology. Formulations were aliquoted into sealed containers and stored under two ICH stability conditions: refrigerated (25°C ± 2°C/60% RH ± 5%) and accelerated (40°C ± 2°C/75% RH ± 5%). Samples were withdrawn monthly for analysis. Particle size and polydispersity index (PDI) were measured by dynamic light scattering (DLS) using a Zetasizer Nano ZS. Zeta potential was measured to assess surface charge and colloidal stability. Drug content was quantified via high-performance liquid chromatography (HPLC) using a validated method for 4-PBA detection. Chemical stability was monitored by Fourier Transform Infrared (FT-IR) spectroscopy to identify peak shifts or disappearance suggestive of degradation or interaction. Differential Scanning Calorimetry (DSC) was used to assess thermal stability by monitoring the preservation of characteristic endothermic events. Visual observations were recorded at each timepoint to detect signs of aggregation, precipitation, or phase separation.
Results: Particle size measurements indicated that N31 remained the most stable among all formulations. At 40°C, N31 particle size increased slightly from 180.4 ± 4.2 nm at Month 0 to 187.6 ± 5.0 nm at Month 3. In comparison, N35 demonstrated significant instability at elevated temperature, with size increasing from 210.5 ± 6.1 nm to 320.8 ± 12.3 nm by Month 3. E2 exhibited moderate increases in size under accelerated conditions but remained relatively stable at 25°C. Microsponge (MS) formulations showed smaller size fluctuations, consistent with their robust porous structure. PDI values for N31 remained consistently below 0.35 across both conditions, indicating uniform size distribution. N35, however, displayed substantial PDI increases under accelerated conditions, rising from 0.38 ± 0.03 at Month 0 to 0.71 ± 0.05 at Month 3, reflecting particle aggregation and loss of uniformity. E2 PDI similarly increased from 0.31 to 0.62 at 40°C, whereas MS PDI values remained relatively moderate across both storage conditions. Zeta potential analysis further confirmed stability trends. N31 maintained a consistent zeta potential of -33.4 ± 2.1 mV, with minimal changes over time under both storage conditions. In contrast, E2 demonstrated a marked reduction in surface charge magnitude under accelerated storage, dropping from -47.8 ± 3.5 mV to -29.5 ± 3.2 mV, suggesting a loss of colloidal stability and a greater propensity for aggregation. MS formulations maintained zeta potentials between -20 and -30 mV, with minimal drift. Drug content analysis showed that N31 preserved over 90% of initial 4-PBA content at both 25°C and 40°C after 3 months. E2 retained between 85% and 88% of its original drug content. N35 demonstrated significant drug degradation at 40°C, retaining only ~65% of its original drug content by Month 3. Microsponge systems maintained approximately 80–85% drug content under both storage conditions. FT-IR spectra confirmed the chemical stability of N31, with no major peak shifts or loss of characteristic bands observed across all timepoints. In contrast, E2 and N35 exhibited minor band shifts and intensity changes near 1600–1700 cm⁻¹ under 40°C conditions, consistent with chemical degradation or molecular rearrangements. Microsponge formulations showed minimal spectral changes. DSC analysis revealed that N31 maintained sharp, distinct endothermic transitions even after 3 months at 40°C, confirming high thermal stability. E2 and N35 lost clear thermal events by Month 2–3 under accelerated conditions, indicating partial degradation and loss of crystalline structure. MS showed moderate thermal broadening but maintained identifiable transition peaks.
Conclusion: N31 chitosan nanoparticles demonstrated superior physicochemical, chemical, and thermal stability under both refrigerated and accelerated storage conditions compared to E2, N35, and MS formulations. N31 retained particle size uniformity, surface charge, drug content, chemical structure, and thermal properties across 3 months of testing. E2 and N35 exhibited significant size increases, PDI broadening, drug content loss, FT-IR peak shifts, and disappearance of thermal transitions under accelerated conditions, suggesting instability. Microsponge formulations showed intermediate stability. Overall, these findings support the selection of N31 as the lead candidate for foam-based decontamination and sustained delivery of 4-PBA for chemical exposure management.
References: 1)Kshirsagar, S.M., Viswaroopan, N., Ghosh, M. et al. Development of 4-phenylbutyric acid microsponge gel formulations for the treatment of lewisite-mediated skin injury. Drug Deliv. and Transl. Res. 15, 638–654 (2025). https://doi.org/10.1007/s13346-024-01620-y
2)Ghosh, M., Viswaroopan, N., Kshirsagar, S. M., Khan, J., Mohiuddin, S., Srivastava, R. K., Athar, M., & Banga, A. K. (2025). Sustained delivery of 4-phenylbutyric acid via chitosan nanoparticles in foam for decontamination and treatment of lewisite-mediated skin injury. International Journal of Pharmaceutics, 682, 125928. https://doi.org/10.1016/j.ijpharm.2025.125928
Acknowledgements: This project was funded by NIH/NIAMS U01AR078544
 Fig 1. Schematic representation of the stability testing workflow for nanoparticle formulations
Fig 1. Schematic representation of the stability testing workflow for nanoparticle formulations Fig 2. (A) FT-IR overlay of E2 nanoparticles stored at 25°C and 40°C over 3 months (B) FT-IR overlay of N35 nanoparticles stored at 25°C and 40°C (C) FT-IR overlay of N31 nanoparticles at 25°C and 40°C (D) FT-IR overlay of MS nanoparticles at 25°C . (E) DSC thermograms of E2 formulations at 40°C for 3 months (F) DSC thermograms of MS formulations at 25°C for 3 months (G) DSC thermograms of N31 nanoparticles at 25°C for 3 months (H) DSC thermograms of N31 nanoparticles at 40°C for 3 months (I) DSC thermograms of N35 nanoparticles at 25°C for 3 months (J) DSC thermograms of N35 nanoparticles at 40°C for 3 months.
Fig 2. (A) FT-IR overlay of E2 nanoparticles stored at 25°C and 40°C over 3 months (B) FT-IR overlay of N35 nanoparticles stored at 25°C and 40°C (C) FT-IR overlay of N31 nanoparticles at 25°C and 40°C (D) FT-IR overlay of MS nanoparticles at 25°C . (E) DSC thermograms of E2 formulations at 40°C for 3 months (F) DSC thermograms of MS formulations at 25°C for 3 months (G) DSC thermograms of N31 nanoparticles at 25°C for 3 months (H) DSC thermograms of N31 nanoparticles at 40°C for 3 months (I) DSC thermograms of N35 nanoparticles at 25°C for 3 months (J) DSC thermograms of N35 nanoparticles at 40°C for 3 months. Fig 3. (A) Particle size of N31, N35, E2, and MS nanoparticles measured over 3 months at 25°C and 40°C storage conditions (B) Zeta potential values of nanoparticle formulations during stability study (C) Polydispersity index (PDI) of nanoparticles across 3 months, indicating changes in size distribution (D) Drug content of 4-PBA in nanoparticles measured monthly by HPLC
Fig 3. (A) Particle size of N31, N35, E2, and MS nanoparticles measured over 3 months at 25°C and 40°C storage conditions (B) Zeta potential values of nanoparticle formulations during stability study (C) Polydispersity index (PDI) of nanoparticles across 3 months, indicating changes in size distribution (D) Drug content of 4-PBA in nanoparticles measured monthly by HPLC