Preclinical, Clinical, and Translational Sciences
(T1230-05-33) Integrated Evaluation of Mechanistic Population PK and Exposure-Response for Dose Justification of Glecirasib in NSCLC Patients with KRAS G12C Mutation
- AX
Alan Jianjun Xiao, Ph.D.
VP, Head of Clinical Pharmacology
Jacobio Pharma US
Burlington, Massachusetts, United States - AX
Alan Jianjun Xiao, Ph.D.
VP, Head of Clinical Pharmacology
Jacobio Pharma US
Burlington, Massachusetts, United States 
Hui Sun, MS
Manager
Jacobio Pharma
BDA, Beijing, China (People's Republic)- XT
Xiaolin Tong, MS
Sr Manager
Jacobio Pharma
BDA, Beijing, China (People's Republic) - AW
Andrea Wang-Gillam, M.D.
CMO
Jacobio Pharma US
Burlington, Massachusetts, United States - YD
Yuli Ding, MS
EVP, Clinical Operations
Jacobio Pharma
BDA, Beijing, China (People's Republic) - ZR
Zhiyue Rao, M.D.
Sr. Director, Medical Science
Jacobio Pharma
BDA, Beijing, China (People's Republic) - CB
Chao Bi, MD
Associate Director, Medical Science
Jacobio Pharma
BDA, Beijing, China (People's Republic) - CQ
Chenglin Qu, MD
Associate Director, Medical Science
Jacobio Pharma
BDA, Beijing, China (People's Republic) - MZ
Mengmeng Zhao, MS
Director, Statistics & Programming
Jacobio Pharma
BDA, Beijing, China (People's Republic) - QL
Qiao Li, Ph.D.
VP, Statistics & Programming
Jacobio Pharma
BDA, Beijing, China (People's Republic) - HW
Haijun Wang, Ph.D.
Sr VP, Information Technology
Jacobio Pharma
BDA, Beijing, China (People's Republic) - YW
Yinxiang Wang, Ph.D.
CEO
Jacobio Pharma
BDA, Beijing, China (People's Republic)
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: Altogether, 4,479 PK samples, including the first pre-dose samples, were collected from 525 patients at five different dose regimens (200, 400 and 800 mg QD, 400 mg BID and 400 mg TID) in two Phase I/II monotherapy studies and two combination studies (one with JAB-3312, and one with cetuximab, anti-EGFR antibody). Glecirasib plasma concentration data from these samples were included in development of glecirasib population PK. Both efficacy data, including tumor size, objective response rate (ORR), progress-free survival (PFS), duration of response (DOR), and overall survival (OS) for 330 patients and safety data, including grade 2+/3+ treatment-related adverse events (TRAE2+/TRAE3+) and grade 2+/3+ treatment emergent adverse events (TEAE2+/TEAE3+) for 342 patients were obtained from the two Phase I/II monotherapy studies. SAS9.4 and R4.4.1 were used for data management and exposure-response analyses. NONMEM v7.5 was used for population PK modeling. NONMEM v7.5 and VBA v7.1 were used for simulation. Glecirasib and JAB-3312 plasma concentration-time data from different studies were integrated for development of their population PK (PPK) models, respectively. The post hoc individual PK parameter estimates of each model were then used to simulate individual concentration data with actual dose information across the whole treatment period to calculate initial (post the first dose), total, time-averaged and steady-state exposure values (Cmax, AUC, Cmin) for each patient, which were then combined with relevant efficacy or safety data to conduct exposure-response analyses. Different mathematic-statistical models were explored for dose/exposure-response analyses, covariate evaluation and sub-group comparisons with different efficacy and safety endpoints.
Results: Glecirasib plasma concentration-time data was well fit with a nonlinear population PK model of two-compartment disposition, a mechanistic solubility-driven absorption plus time-dependent bioavailability and clearance, which to our knowledge, is the first time published in the class of KRAS mutation inhibitors[1]. A representative time course of daily, total and time-averaged exposure (e.g. AUC) was illustrated in Figure 1. Patient demographics, disease status, and prior therapies were evaluated but none was identified as a clinically meaningful factor to impact glecirasib exposure. A significant dose/exposure-response relationship was identified between total AUC and multiple efficacy endpoints including: tumor size %change from baseline (TSpcfb), best objective response rate (BORR, defined as TSpcfb≤-30%), independent review committee/principal investigator (IRC/PI) confirmed ORR, PFS, OS and DOR. Out of multiple risk factors of interest, baseline tumor burden was identified as a significant risk factor to negatively impact ORR, PFS and OS. This is the first time to report a significant exposure-response relationship in the class of KRAS mutation inhibitors. More importantly, the methods and findings in this evaluation can also explain the previously observed but uninterpretable inverse exposure-response relationship for sotorasib[2], an FDA approved KRAS G12C inhibitor. With a direct dose-to-dose comparison, relative to 400 mg QD, 800 mg QD in NSCLC patients had a better BORR (57.3% vs 40.0%), IRC/PI confirmed ORR (47.6% vs 31.4%), PFS (8.3 vs 5.6 months), OS (15.5 vs 12.0 months) and DOR (13.9 vs 6.8 months), respectively. Figure 2 and 3 showed two representative relationships. In the safety analysis, no significant dose/exposure-response relationship was identified for grade 2+ TRAE, grade 3+ TRAE or grade 2+ TEAE. A direct dose-to-dose Pearson’s Chi-square test and Fisher’s Exact test demonstrated no significant difference for these 3 endpoints between the 400 mg QD and 800 mg QD dose groups, either. Although a significant relationship was identified between grade 3+ TEAE rate and dose/first-day Cmax, along with a significant difference in this rate between 400 mg QD and 800 mg QD, all AEs were manageable, and both doses were considered well tolerated.
Conclusion: Glecirasib PK were well characterized by a mechanistic population PK model, and significant exposure-response relationships were identified for all major efficacy endpoints, first time for such findings reported in the class of KRAS mutation inhibitors. Based on the best overall benefit/risk balance, the selection of 800 mg QD glecirasib for treatment of NSCLC with KRAS G12C mutation was well justified.
References: 1. Marlo Nagase, Brett Houk, Irene Vuu et al, Population Pharmacokinetics of Sotorasib in Healthy Subjects and Advanced Solid Tumor Patients Harboring a KRASG12C Mutation from Phase 1 and Phase 2 Studies. The AAPS Journal (2025) 27:26. https://doi.org/10.1208/s12248-024-01013-6.
2. FDA CDER, NDA/BLA Multi-disciplinary Review and Evaluation {NDA 214665} for LUMAKRASTM (sotorasib). Jan 2020.
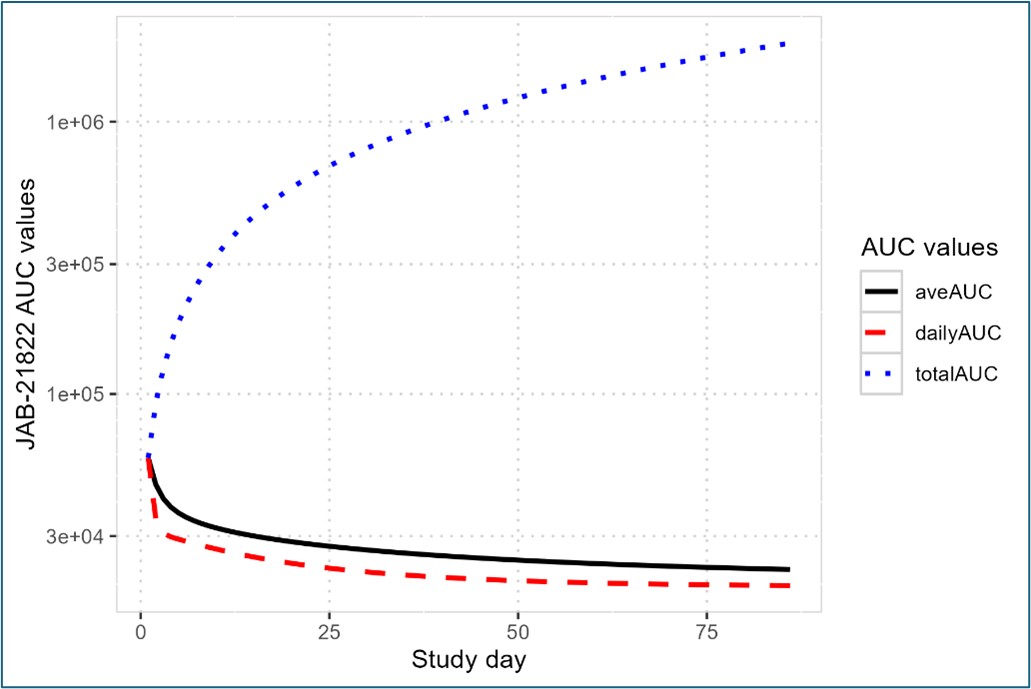 Figure 1. Glecirasib Population-PK Model-predicted Typical Time-course of Glecirasib AUC values at 800 mg QD in NSCLC Patients.
Figure 1. Glecirasib Population-PK Model-predicted Typical Time-course of Glecirasib AUC values at 800 mg QD in NSCLC Patients. Note: in legend, aveAUC for time-averaged AUC; dailyAUC for daily AUC and totalAUC for time accumulated total AUC.
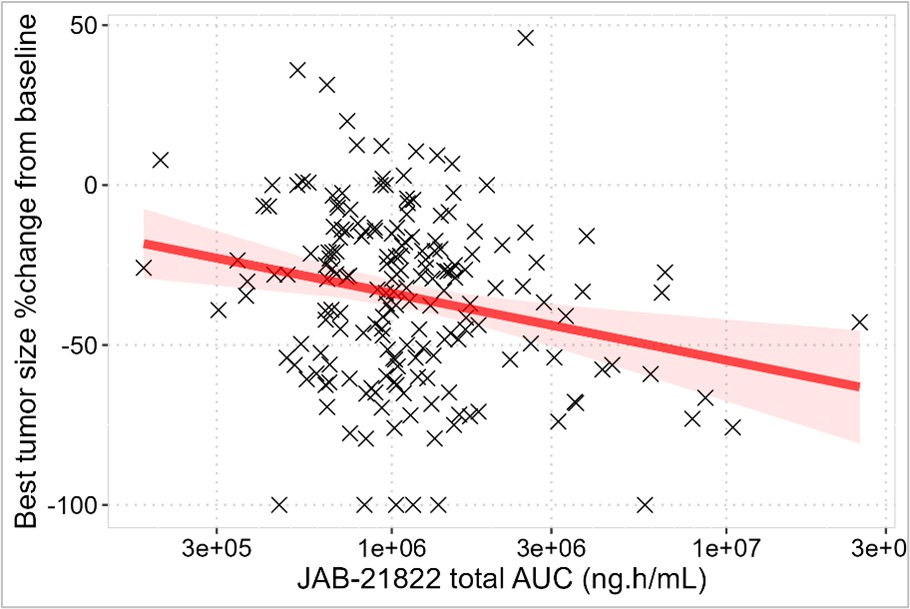 Figure 2. Best Tumor Size %Change from Baseline vs Glecirasib Total AUC in NSCLC Patients.
Figure 2. Best Tumor Size %Change from Baseline vs Glecirasib Total AUC in NSCLC Patients. Note: symbols for measurements; lines/shades for model-predict and 95%CI.
Model formula in R: y ~ c0 + c1 * log10(x).
Parameters: Estimate, Std. Error, t value, Pr(>|t|).
c0: 91.933, 39.340, 2.337, 0.02052 * .
c1: -20.958, 6.494, -3.227, 0.00148 **.
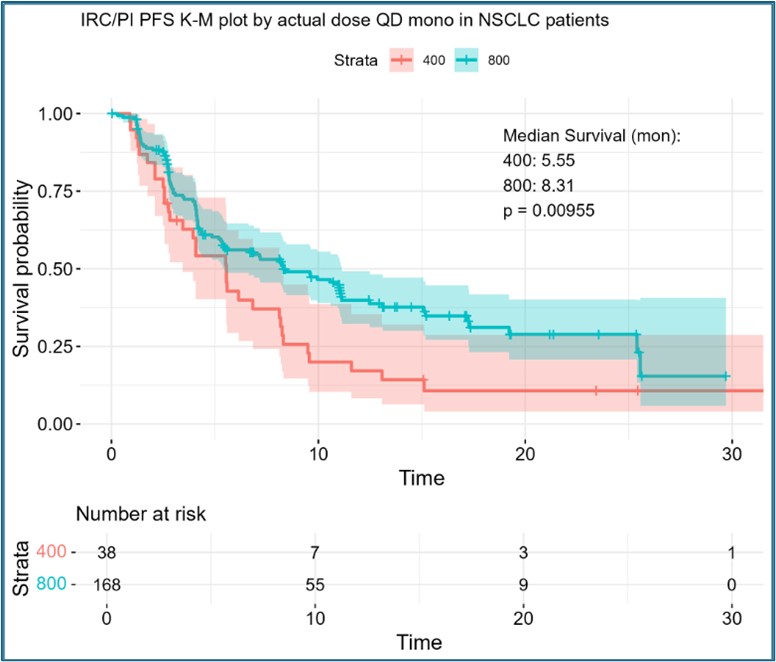 Figure 3. IRC/PI Confirmed PFS Kaplan-Meier Survival at Glecirasib 400 vs 800 mg QD.
Figure 3. IRC/PI Confirmed PFS Kaplan-Meier Survival at Glecirasib 400 vs 800 mg QD.Note: lines/shaded areas for K-M survival curve and 95%CI.
