Formulation and Delivery - Chemical
(W0930-09-57) In Vitro Bioequivalence Evaluation of Topical Creams Throughout the Product’s In-Use Shelf Life
- SK
Sumedha Kapre, MS
Graduate Teaching Assistant
Texas A&M University
Kingsville, Texas, United States - SK
Sumedha Kapre, MS
Graduate Teaching Assistant
Texas A&M University
Kingsville, Texas, United States - SP
Sushesh Srivatsa Palakurthi, MS
Ph.D. Candidate
Texas A&M University
Kingsville, Texas, United States - MT
Maharshi Thalla, Ph.D.
Postdoctoral Research Associate
Texas A&M University
Kingsville, Texas, United States - NA
Narangerel Altangerel, Ph.D.
Postdoctoral Research Associate
Texas A&M University
College Station, Texas, United States - SP
Sarea Phillips, MS
Graduate Student
Texas A&M University
College Station, Texas, United States - SP
Srinath Palakurthi, Ph.D. (he/him/his)
Professor
Texas A&M University
Kingsville, Texas, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: Three formulations, C1 (Reference) and C2 & C3 (Test), were comprehensively evaluated. Rheological assessments, encompassing amplitude, time, temperature, and frequency sweeps, were conducted using an MCR 301 rheometer (Anton Paar) to elucidate the viscoelastic properties of the creams. Compression tests were performed at room temperature on the formulations using a Texture Analyzer TA. XT Plus (Stable Micro Systems, UK). Globule size distribution was determined using an Anton Paar Particle Size Analyzer. IVPT was performed using excised porcine ear skin in vertical Franz diffusion cells at 32 ± 0.5 °C over a 24-hour period, with cumulative amount permeated and flux subsequently determined. Confocal Raman microscopy (LabRAM HR Evolution - HORIBA), equipped with a 662 nm laser, was employed to obtain high-resolution images of active pharmaceutical ingredient (API) distribution within the skin at various time points over 24 hours. All studies were conducted at Day 1, Day 45, and Day 90 to encompass the entire in-use shelf life of the product post-opening.
Results: Texture analysis performed at Day 90 revealed significant differences in the mechanical behavior of the topical creams. While compression force remained consistent across all three creams (~7000 g), cohesiveness and adhesiveness varied notably. C1 exhibited the highest adhesiveness (~5000 g), whereas C2 and C3 demonstrated similar, lower adhesive forces (~2000 g). Rheological assessment indicated critical differences in the storage modulus, with C1 displaying the highest shear storage modulus (~3000 Pa) compared to C2 and C3 (~2000 Pa). Globule size distribution measured at Day 90 showed a clear distinction: C1 (1.91 µm) differed significantly from C2 (59.42 µm) and C3 (87.15 µm). No change in the melting point of the API was observed in the DSC thermograms recorded at Day 90. Furthermore, significant differences in permeation profiles and flux were observed in the IVPT study at Day 90, with C1 demonstrating the highest permeation and flux (11.47±1.85 µg/cm2/h). The flux values for C2 and C3 were comparable, at 4.81±5.98 and 5.95±1.82 µg/cm2/h respectively. Preliminary confocal Raman imaging conducted at Day 90 revealed distinct differences in the C1 cream when imaged on a microscopic slide and within the skin. Specifically, critical peaks in the fingerprint region (850-1150 cm⁻¹) observed when imaging the creams directly, and a unique peak at 1300 cm⁻¹ observed for C1 within the skin, highlight key distinctions of C1 compared to the test creams.
Conclusion: This study characterized the physicochemical and structural properties of pimecrolimus creams throughout their in-use shelf life. Significant differences were consistently observed across texture, rheological, and globule size profiles, with similar trends noted in IVPT and preliminary confocal Raman imaging. The quantification of fingerprint region peaks from Raman spectra and the validation of the developed IVPT and Raman imaging techniques hold substantial promise. These methods collectively represent a potential sequence of in vitro characterization techniques for comprehensively assessing Q1/Q2 and Q3 in vitro bioequivalence of topical creams
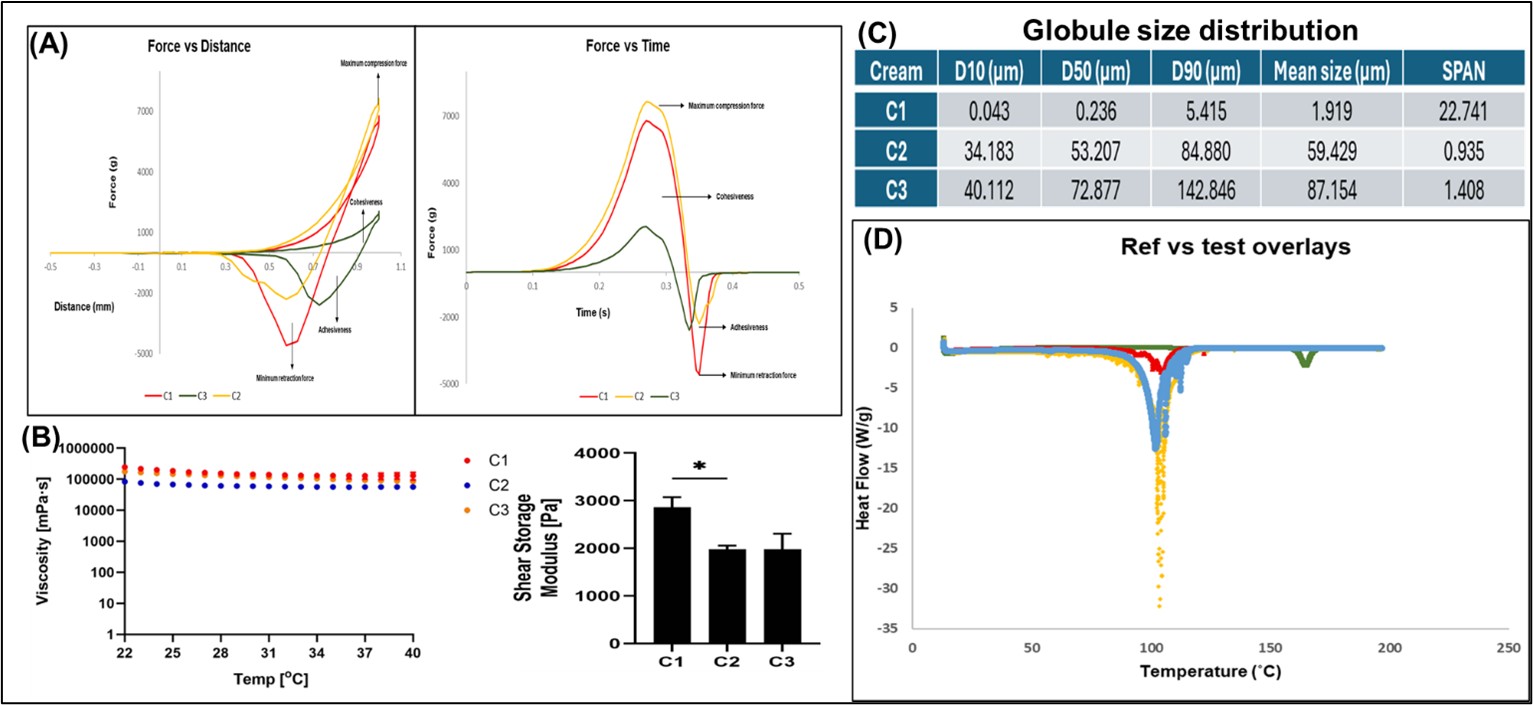 Figure 1: Physicochemical and structural characterization of pimecrolimus 1% creams. (A) Texture analysis of the creams was performed at room temperature (RT) using an XT Plus texture analyzer. Adhesive, cohesive, and mechanical forces were assessed and plotted as force vs. distance and force vs. time.
Figure 1: Physicochemical and structural characterization of pimecrolimus 1% creams. (A) Texture analysis of the creams was performed at room temperature (RT) using an XT Plus texture analyzer. Adhesive, cohesive, and mechanical forces were assessed and plotted as force vs. distance and force vs. time.(B) Rheological properties were evaluated using an Anton Paar MCR 301 rheometer. The effect of temperature on viscosity and changes in storage modulus (G') of the creams were studied.
(C) Globule size distribution was analyzed using an Anton Paar dynamic light scattering (DLS) particle size analyzer. A measured quantity of each cream was diluted 10-fold (0.1%) with distilled water prior to analysis.
(D) Differential scanning calorimetry (DSC) thermograms were recorded using a TA Instruments Q2000 DSC. Approximately 5 mg of cream was placed in a hermetically sealed DSC pan. Temperature scans were conducted from 2 °C to 200 °C with a ramp rate of 10 °C. All experiments were conducted in triplicate (n = 3)
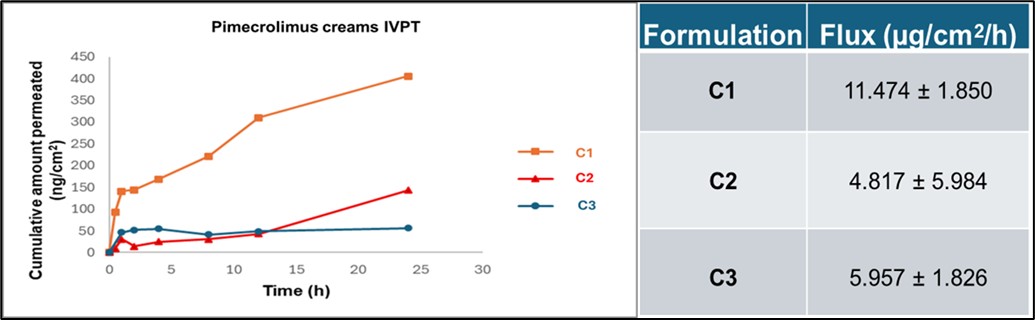 Figure 2: In vitro permeation studies were conducted using vertical Franz diffusion cells with a receptor volume of 5 mL and a donor surface area of 0.2 cm². The receptor medium consisted of 1× phosphate-buffered saline (PBS) containing 30% Transcutol® HP, pH 7.2. Porcine ear skin was prepared, cut to uniform thickness, and mounted between the donor and receptor compartments. The experiment was carried out for 24 hours at 32 ± 0.5 °C. Samples were collected at predetermined time intervals and analyzed for pimecrolimus content. All experiments were performed in triplicate (n = 3), and results are reported as cumulative amount permeated (ng/cm²) versus time. Steady-state flux values were calculated and expressed as mean flux ± standard deviation (S.D.).
Figure 2: In vitro permeation studies were conducted using vertical Franz diffusion cells with a receptor volume of 5 mL and a donor surface area of 0.2 cm². The receptor medium consisted of 1× phosphate-buffered saline (PBS) containing 30% Transcutol® HP, pH 7.2. Porcine ear skin was prepared, cut to uniform thickness, and mounted between the donor and receptor compartments. The experiment was carried out for 24 hours at 32 ± 0.5 °C. Samples were collected at predetermined time intervals and analyzed for pimecrolimus content. All experiments were performed in triplicate (n = 3), and results are reported as cumulative amount permeated (ng/cm²) versus time. Steady-state flux values were calculated and expressed as mean flux ± standard deviation (S.D.).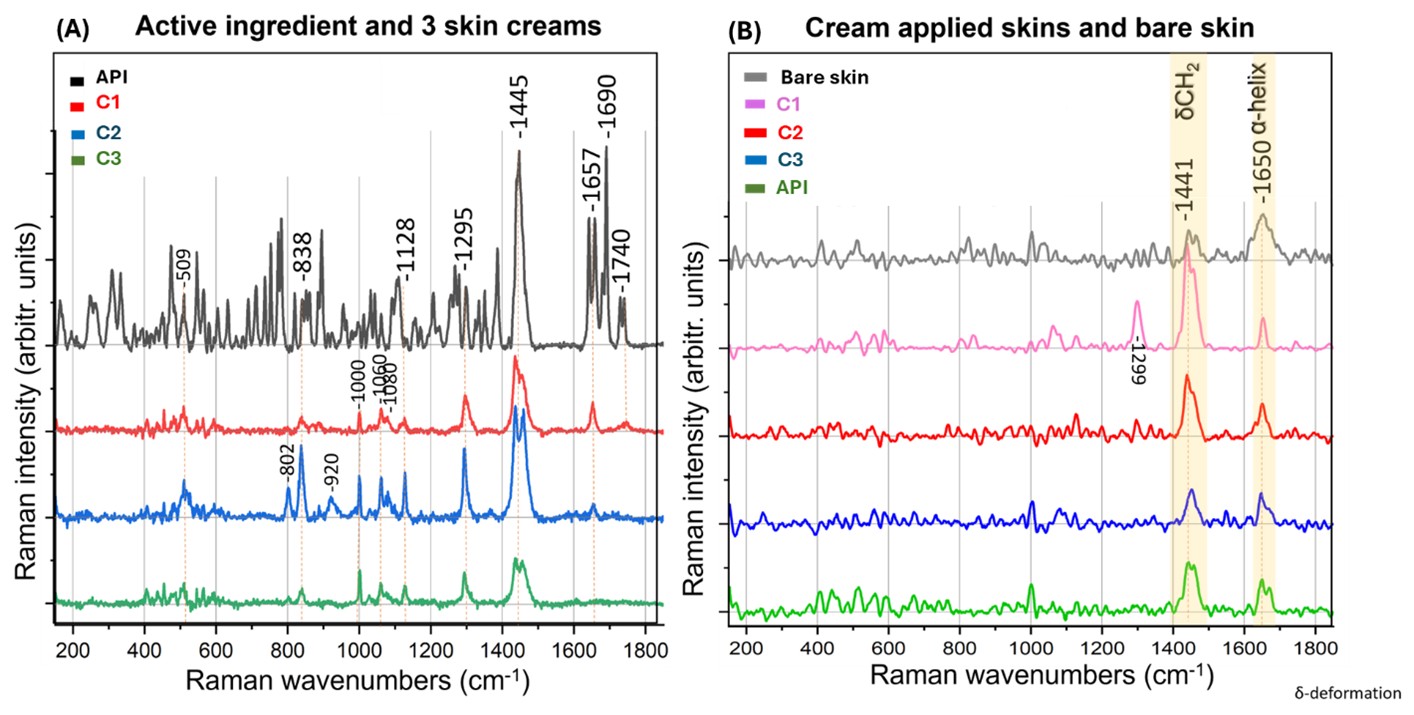 Figure 3: Representative Raman spectra were obtained using a confocal Raman microscope (LabRAM HR Evolution, HORIBA) within the fingerprint region (150–1850 cm⁻¹). Samples of pimecrolimus, as well as cream formulations C1, C2, and C3, were imaged both on microscope slides and on porcine ear skin membranes.
Figure 3: Representative Raman spectra were obtained using a confocal Raman microscope (LabRAM HR Evolution, HORIBA) within the fingerprint region (150–1850 cm⁻¹). Samples of pimecrolimus, as well as cream formulations C1, C2, and C3, were imaged both on microscope slides and on porcine ear skin membranes.