Discovery and Basic Research
(W1230-03-19) Pharmacokinetics and Biodistribution of FVIII Containing LysoPS Nanoparticle Following IV Administration
Wednesday, November 12, 2025
12:30 PM - 1:30 PM CT
- MP
Manali Patel, MS
PhD Candidate
University at Buffalo
Buffalo, New York, United States - MP
Manali Patel, MS
PhD Candidate
University at Buffalo
Buffalo, New York, United States 
Vincent Chak, PhD (he/him/his)
PhD Candidate
University at Buffalo
Buffalo, New York, United States- BS
Beverly Schaefer, M.D.
Medical Director
WNYBloodCare (formerly Hemophilia Center of WNY)
buffalo, New York, United States 
Sathy V. Balu-Iyer, Ph.D.
Professor
University at Buffalo
Buffalo, New York, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Replacement therapy with recombinant Factor VIII (FVIII) is the first-line treatment for Hemophilia A (HA), a congenital bleeding disorder. However, its effectiveness is limited by rapid clearance and the development of inhibitors, requiring frequent high-dose infusions. There is an unmet need for FVIII formulations that not only extend half-life but also promote immune tolerance. To address this, we developed a Lyso-phosphatidylserine (LysoPS)-containing lipid nanoparticle platform that leverages the immunomodulatory properties of phosphatidylserine to transform FVIII from an immunogen into a tolerogen. To investigate whether association with the LysoPS nanoparticle prolongs plasma survival of FVIII, comparative PK studies were conducted at the clinically relevant doses along with hemostatic efficacy studies in HA mice. PK modeling and informed scaling approach were applied to assess the translatability of LysoPS-FVIII to humans. Additionally, biodistribution studies were conducted to track the fate of empty LysoPS nanoparticles following IV administration in mice.
Methods: Pharmacokinetics: Hemophilia A (HA) mice (C57BL/6J) received a single retro-orbital injection of either free FVIII or LysoPS-FVIII at various clinically relevant doses (20, 40, and 80 IU/kg). Blood was collected up to 78 hours post-injection (n=3 per time point/dose) and FVIII activity was measured by chromogenic assay and compared to standards of known activity. Basic PK parameters (CL, t½, AUC, MRT) were calculated using non-compartmental analysis method. Model fitting was performed using ADAPT 5 and goodness-of-fit was assessed with a combination of analytical criteria and visual predictive plots. Pharmacodynamics: Ex vivo hemostatic efficacy at time points corresponding to PK study for 40 IU/kg dose was measured using activated partial thromboplastin time (aPTT) assay. Scaling PK: An informed scaling approach combining allometric PK parameter scaling with normalized Wajima curves was used to simulate behavior of LysoPS-FVIII in humans (1). Biodistribution studies: Swiss Webster mice were injected with either free indocyanine green (ICG) or ICG-loaded LysoPS nanoparticles for whole-body imaging. Organs were collected at 1- and 3-hours post-injection and imaged using the FMT 2000 In Vivo Imaging System. Liver localization was further confirmed using confocal microscopy analysis post retro-orbital injection of Dil dye-labeled LysoPS nanoparticles.
Results: Higher plasma levels of LysoPS-FVIII were seen within the first 24 hours, as compared to free FVIII groups (Fig1A). Noncompartmental analyses indicated that LysoPS association decreases the clearance and prolongs plasma survival of FVIII. Estimated AUC was 1.5-fold greater for LysoPS-FVIII compared to free FVIII, suggesting increase in overall systemic exposure (Table 1). LysoPS-FVIII demonstrated enhanced hemostatic efficacy with significantly shorter aPTT clotting times (Fig1B). Combined PK/PD data indicate that LysoPS extends hemostatic efficacy by maintaining FVIII levels above 0.01 IU/mL for a longer duration compared to free FVIII (Fig1C, D). A final 2-compartment model with linear elimination model best described the data in mice (Fig2A). Simulations for humans indicate association with LysoPS nanoparticles is expected to prolong circulating half-life of FVIII from 14.8 to 19.5 h. Time to reach minimum therapeutic threshold of 0.01 IU/ml also increased from 3.5 to 4.5 days in humans (Fig2B). In whole-body imaging, LysoPS-ICG accumulated in the liver within 1 hour, unlike free ICG, and was cleared by 3 hours after administration (Fig3A). From collected organs, LysoPS-ICG treated mice showed strongest fluorescence signal in the liver, suggesting liver is the major organ for LysoPS disposition (Fig3B). Further confocal imaging of liver tissues from mice treated with LysoPS-DiI nanoparticles confirmed strong fluorescence throughout liver tissue compared to control (Fig3C, D, E).
Conclusion: LysoPS-FVIII enhances FVIII plasma persistence without compromising PK over a range of doses, consistent with prior findings that lipid binding improves FVIII survival (2). Simulations for humans predict a 32% increase in half-life over free FVIII making it a viable delivery strategy in humans. LysoPS may be capable of prolonging the time to reach a minimum trough concentration of 0.01 IU/mL by a full day. High fluorescence intensity in the liver suggests that the liver could be the tolerogenic site for LysoPS-mediated tolerance. Together, LysoPS nanoparticle is a multifunctional platform that improves FVIII therapy by extending circulation and has potential of promoting immune tolerance in hemophilia A.
References: 1. Kosloski MP, Pisal DS, Mager DE, Balu-Iyer SV. Allometry of factor VIII and informed scaling of next-generation therapeutic proteins. J Pharm Sci. 2013;102(7):2380-94.
2. Pisal DS, Balu-Iyer SV. Phospholipid binding improves plasma survival of factor VIII. Thromb Haemost. 2010;104(5):1073-5.
Acknowledgements: Funding was provided by the NHI/NHLBI R61HL161818 to SVB, RO1 Al169296. The authors would like to acknowledge the UB Optical Imaging and Analysis Facility for the use of the confocal fluorescence imaging. Dr. Dhaval Shah for the use of FMT 2000 In Vivo Imaging System. We are grateful to the Western New York BloodCare for providing the recombinant FVIII products.
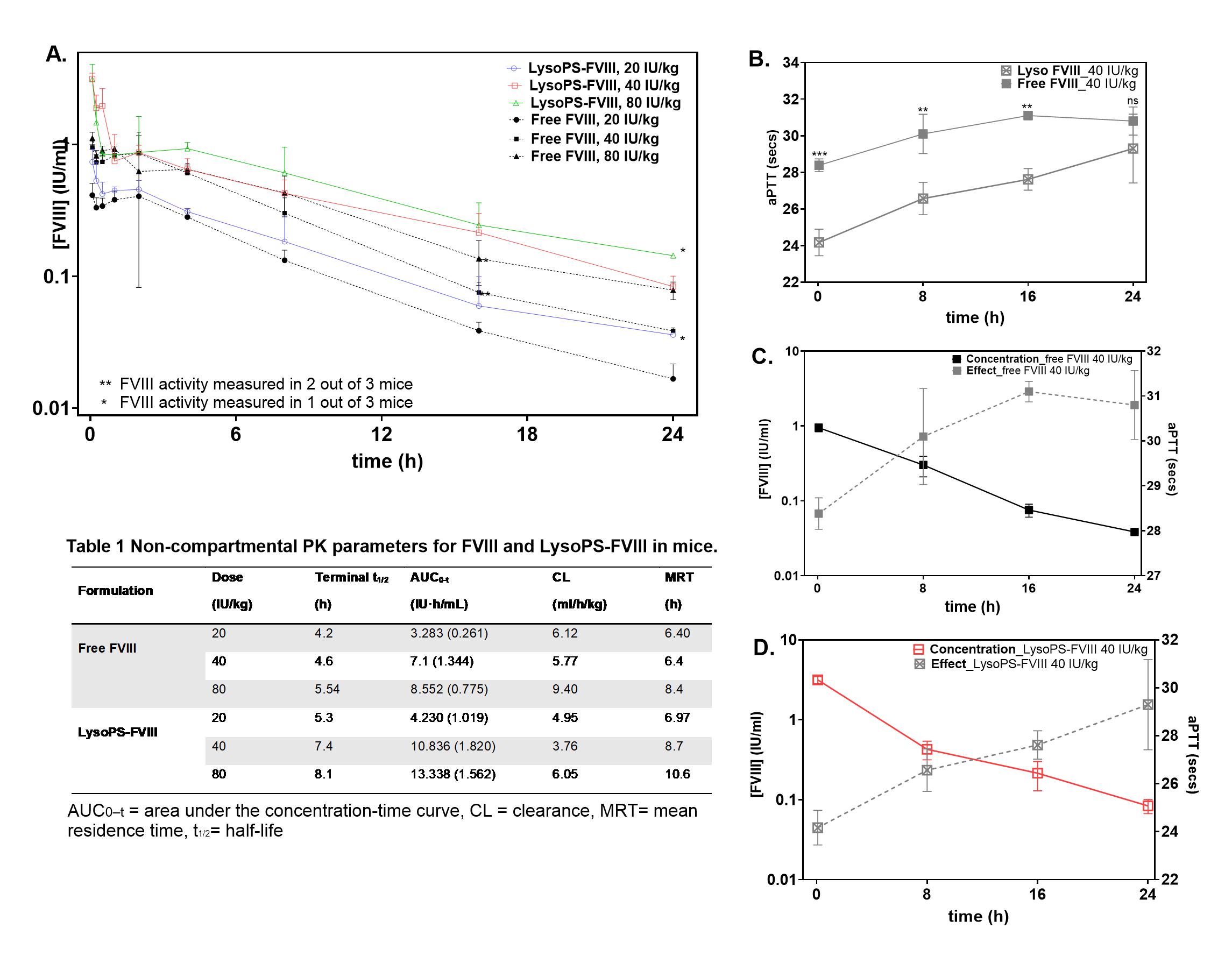 Fig 1: Pharmacokinetics profiles of FVIII and LysoPS-FVIII in HA mice. A) Plasma concentration-time profiles following IV administration of free FVIII at 20 (closed circles), 40 (closed square), or 80 (closed triangle) IU/kg or LysoPS-FVIII at 20 (open circles), 40 (open square), or 80 (open triangle) IU/kg. Table 1: Pharmacokinetic parameters determined by non-compartmental analysis. B) Ex vivo hemostatic efficacy measured using activated partial thromboplastin time (aPTT) assay in HA mice at dose 40IU/kg. Statistics were performed using two-way ANOVA-Bonferroni’s multiple comparison test (*P < 0.05). Overlay of PK and PD profiles after C) free FVIII treatment D) after LysoPS-FVIII.
Fig 1: Pharmacokinetics profiles of FVIII and LysoPS-FVIII in HA mice. A) Plasma concentration-time profiles following IV administration of free FVIII at 20 (closed circles), 40 (closed square), or 80 (closed triangle) IU/kg or LysoPS-FVIII at 20 (open circles), 40 (open square), or 80 (open triangle) IU/kg. Table 1: Pharmacokinetic parameters determined by non-compartmental analysis. B) Ex vivo hemostatic efficacy measured using activated partial thromboplastin time (aPTT) assay in HA mice at dose 40IU/kg. Statistics were performed using two-way ANOVA-Bonferroni’s multiple comparison test (*P < 0.05). Overlay of PK and PD profiles after C) free FVIII treatment D) after LysoPS-FVIII.
.jpg) Fig 2: Simulated clinical PK profiles and projected impact of LysoPS association on prophylactic free FVIII administration. A) Model fitting for LysoPS-FVIII using a 2-compartment linear clearance model (solid lines). Model fitted parameters for LysoPS-FVIII (Table 2). B) Projected human PK profile of LysoPS-FVIII (red) and observed disposition of free FVIII in humans (black) at 40 IU/kg doses. Literature-derived parameters were used for free FVIII simulations, while scaled human PK parameters were predicted using an “informed scaling” approach combining allometric PK parameter scaling with normalized Wajima curves (Table 3).
Fig 2: Simulated clinical PK profiles and projected impact of LysoPS association on prophylactic free FVIII administration. A) Model fitting for LysoPS-FVIII using a 2-compartment linear clearance model (solid lines). Model fitted parameters for LysoPS-FVIII (Table 2). B) Projected human PK profile of LysoPS-FVIII (red) and observed disposition of free FVIII in humans (black) at 40 IU/kg doses. Literature-derived parameters were used for free FVIII simulations, while scaled human PK parameters were predicted using an “informed scaling” approach combining allometric PK parameter scaling with normalized Wajima curves (Table 3).
.jpg) Figure 3: Biodistribution studies A) Whole-body imaging post retro orbital injection of either free indocyanine green (ICG) or ICG-loaded LysoPS nanoparticles in Swiss Webster mice. B) Organs were collected at 1- and 3-hours post-injection and imaged using the FMT 2000 In Vivo Imaging System. Liver localization was further confirmed using confocal microscopy analysis post retro-orbital injection of C) buffer D) Dil dye-labeled LysoPS nanoparticles (DAPI-Blue, Liposome- Yellow, LYVE1- Green) E) Comparison of Mean fluorescence intensity (MFI).
Figure 3: Biodistribution studies A) Whole-body imaging post retro orbital injection of either free indocyanine green (ICG) or ICG-loaded LysoPS nanoparticles in Swiss Webster mice. B) Organs were collected at 1- and 3-hours post-injection and imaged using the FMT 2000 In Vivo Imaging System. Liver localization was further confirmed using confocal microscopy analysis post retro-orbital injection of C) buffer D) Dil dye-labeled LysoPS nanoparticles (DAPI-Blue, Liposome- Yellow, LYVE1- Green) E) Comparison of Mean fluorescence intensity (MFI).
Methods: Pharmacokinetics: Hemophilia A (HA) mice (C57BL/6J) received a single retro-orbital injection of either free FVIII or LysoPS-FVIII at various clinically relevant doses (20, 40, and 80 IU/kg). Blood was collected up to 78 hours post-injection (n=3 per time point/dose) and FVIII activity was measured by chromogenic assay and compared to standards of known activity. Basic PK parameters (CL, t½, AUC, MRT) were calculated using non-compartmental analysis method. Model fitting was performed using ADAPT 5 and goodness-of-fit was assessed with a combination of analytical criteria and visual predictive plots. Pharmacodynamics: Ex vivo hemostatic efficacy at time points corresponding to PK study for 40 IU/kg dose was measured using activated partial thromboplastin time (aPTT) assay. Scaling PK: An informed scaling approach combining allometric PK parameter scaling with normalized Wajima curves was used to simulate behavior of LysoPS-FVIII in humans (1). Biodistribution studies: Swiss Webster mice were injected with either free indocyanine green (ICG) or ICG-loaded LysoPS nanoparticles for whole-body imaging. Organs were collected at 1- and 3-hours post-injection and imaged using the FMT 2000 In Vivo Imaging System. Liver localization was further confirmed using confocal microscopy analysis post retro-orbital injection of Dil dye-labeled LysoPS nanoparticles.
Results: Higher plasma levels of LysoPS-FVIII were seen within the first 24 hours, as compared to free FVIII groups (Fig1A). Noncompartmental analyses indicated that LysoPS association decreases the clearance and prolongs plasma survival of FVIII. Estimated AUC was 1.5-fold greater for LysoPS-FVIII compared to free FVIII, suggesting increase in overall systemic exposure (Table 1). LysoPS-FVIII demonstrated enhanced hemostatic efficacy with significantly shorter aPTT clotting times (Fig1B). Combined PK/PD data indicate that LysoPS extends hemostatic efficacy by maintaining FVIII levels above 0.01 IU/mL for a longer duration compared to free FVIII (Fig1C, D). A final 2-compartment model with linear elimination model best described the data in mice (Fig2A). Simulations for humans indicate association with LysoPS nanoparticles is expected to prolong circulating half-life of FVIII from 14.8 to 19.5 h. Time to reach minimum therapeutic threshold of 0.01 IU/ml also increased from 3.5 to 4.5 days in humans (Fig2B). In whole-body imaging, LysoPS-ICG accumulated in the liver within 1 hour, unlike free ICG, and was cleared by 3 hours after administration (Fig3A). From collected organs, LysoPS-ICG treated mice showed strongest fluorescence signal in the liver, suggesting liver is the major organ for LysoPS disposition (Fig3B). Further confocal imaging of liver tissues from mice treated with LysoPS-DiI nanoparticles confirmed strong fluorescence throughout liver tissue compared to control (Fig3C, D, E).
Conclusion: LysoPS-FVIII enhances FVIII plasma persistence without compromising PK over a range of doses, consistent with prior findings that lipid binding improves FVIII survival (2). Simulations for humans predict a 32% increase in half-life over free FVIII making it a viable delivery strategy in humans. LysoPS may be capable of prolonging the time to reach a minimum trough concentration of 0.01 IU/mL by a full day. High fluorescence intensity in the liver suggests that the liver could be the tolerogenic site for LysoPS-mediated tolerance. Together, LysoPS nanoparticle is a multifunctional platform that improves FVIII therapy by extending circulation and has potential of promoting immune tolerance in hemophilia A.
References: 1. Kosloski MP, Pisal DS, Mager DE, Balu-Iyer SV. Allometry of factor VIII and informed scaling of next-generation therapeutic proteins. J Pharm Sci. 2013;102(7):2380-94.
2. Pisal DS, Balu-Iyer SV. Phospholipid binding improves plasma survival of factor VIII. Thromb Haemost. 2010;104(5):1073-5.
Acknowledgements: Funding was provided by the NHI/NHLBI R61HL161818 to SVB, RO1 Al169296. The authors would like to acknowledge the UB Optical Imaging and Analysis Facility for the use of the confocal fluorescence imaging. Dr. Dhaval Shah for the use of FMT 2000 In Vivo Imaging System. We are grateful to the Western New York BloodCare for providing the recombinant FVIII products.
 Fig 1: Pharmacokinetics profiles of FVIII and LysoPS-FVIII in HA mice. A) Plasma concentration-time profiles following IV administration of free FVIII at 20 (closed circles), 40 (closed square), or 80 (closed triangle) IU/kg or LysoPS-FVIII at 20 (open circles), 40 (open square), or 80 (open triangle) IU/kg. Table 1: Pharmacokinetic parameters determined by non-compartmental analysis. B) Ex vivo hemostatic efficacy measured using activated partial thromboplastin time (aPTT) assay in HA mice at dose 40IU/kg. Statistics were performed using two-way ANOVA-Bonferroni’s multiple comparison test (*P < 0.05). Overlay of PK and PD profiles after C) free FVIII treatment D) after LysoPS-FVIII.
Fig 1: Pharmacokinetics profiles of FVIII and LysoPS-FVIII in HA mice. A) Plasma concentration-time profiles following IV administration of free FVIII at 20 (closed circles), 40 (closed square), or 80 (closed triangle) IU/kg or LysoPS-FVIII at 20 (open circles), 40 (open square), or 80 (open triangle) IU/kg. Table 1: Pharmacokinetic parameters determined by non-compartmental analysis. B) Ex vivo hemostatic efficacy measured using activated partial thromboplastin time (aPTT) assay in HA mice at dose 40IU/kg. Statistics were performed using two-way ANOVA-Bonferroni’s multiple comparison test (*P < 0.05). Overlay of PK and PD profiles after C) free FVIII treatment D) after LysoPS-FVIII..jpg) Fig 2: Simulated clinical PK profiles and projected impact of LysoPS association on prophylactic free FVIII administration. A) Model fitting for LysoPS-FVIII using a 2-compartment linear clearance model (solid lines). Model fitted parameters for LysoPS-FVIII (Table 2). B) Projected human PK profile of LysoPS-FVIII (red) and observed disposition of free FVIII in humans (black) at 40 IU/kg doses. Literature-derived parameters were used for free FVIII simulations, while scaled human PK parameters were predicted using an “informed scaling” approach combining allometric PK parameter scaling with normalized Wajima curves (Table 3).
Fig 2: Simulated clinical PK profiles and projected impact of LysoPS association on prophylactic free FVIII administration. A) Model fitting for LysoPS-FVIII using a 2-compartment linear clearance model (solid lines). Model fitted parameters for LysoPS-FVIII (Table 2). B) Projected human PK profile of LysoPS-FVIII (red) and observed disposition of free FVIII in humans (black) at 40 IU/kg doses. Literature-derived parameters were used for free FVIII simulations, while scaled human PK parameters were predicted using an “informed scaling” approach combining allometric PK parameter scaling with normalized Wajima curves (Table 3)..jpg) Figure 3: Biodistribution studies A) Whole-body imaging post retro orbital injection of either free indocyanine green (ICG) or ICG-loaded LysoPS nanoparticles in Swiss Webster mice. B) Organs were collected at 1- and 3-hours post-injection and imaged using the FMT 2000 In Vivo Imaging System. Liver localization was further confirmed using confocal microscopy analysis post retro-orbital injection of C) buffer D) Dil dye-labeled LysoPS nanoparticles (DAPI-Blue, Liposome- Yellow, LYVE1- Green) E) Comparison of Mean fluorescence intensity (MFI).
Figure 3: Biodistribution studies A) Whole-body imaging post retro orbital injection of either free indocyanine green (ICG) or ICG-loaded LysoPS nanoparticles in Swiss Webster mice. B) Organs were collected at 1- and 3-hours post-injection and imaged using the FMT 2000 In Vivo Imaging System. Liver localization was further confirmed using confocal microscopy analysis post retro-orbital injection of C) buffer D) Dil dye-labeled LysoPS nanoparticles (DAPI-Blue, Liposome- Yellow, LYVE1- Green) E) Comparison of Mean fluorescence intensity (MFI).