Formulation and Delivery - Biomolecular
(W1230-08-48) Beyond Specificity: Evaluating the Cross-Protective Potential of a Whole-Cell Inactivated Microparticulate Gonorrhea Vaccine
- AF
Amarae Ferguson, MS (he/him/his)
Research Assistant
Mercer University
Atlanta, Georgia, United States - AF
Amarae Ferguson, MS (he/him/his)
Research Assistant
Mercer University
Atlanta, Georgia, United States - PB
Priyal Vishnu Bagwe, Ph.D.
Postdoctoral Scientist
Merck & Co., Inc.
Cambridge, Massachusetts, United States 
Dedeepya Pasupuleti, PhD (she/her/hers)
Faculty
Larkin University
Miami, Florida, United States- TA
Tanisha Manoj Arte, MS
PhD Student
Mercer University
Atlanta, Georgia, United States 
Emmanuel Adediran, BS
PhD Candidate
Mercer University
Atlanta, Georgia, United States- MG
Mahek Gulani, BS
PhD Student
Mercer University
Atlanta, Georgia, United States 
Susu Zughaier, PhD (she/her/hers)
Associate Professor of Microbiology and Immunology
Qatar University
Atlanta, Georgia, United States
Martin J. D’Souza, Ph.D. (he/him/his)
Professor
Mercer University
Atlanta, Georgia, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Methods: The Neisseria gonorrhoeae was first grown and then formalin-inactivated. Then, using a method developed in our lab, we encapsulated the whole cell inactivated gonorrhea into a biodegradable pre-crosslinked albumin microparticles along with the adjuvants Alum and AddaVax™ using spray drying method. Next, these microparticles were loaded into dissolving microneedles made from hyaluronic acid, deionized water, and trehalose. Then, five groups were tested: a naïve control (A), a group vaccinated with CDC F62 vaccine and challenged with CDC F62 (B), a group vaccinated with adjuvanted CDC F62 vaccine and challenged with CDC F62 (C), a group vaccinated with adjuvanted CDC F62 vaccine and challenged with the heterologous FA19 strain (D), and a group vaccinated with adjuvanted CDC F62 vaccine and challenged with the heterologous FA1090 strain (E). Every two weeks, we collected blood samples. Then, at week 8, we challenged the groups with the different strains of gonorrhea to evaluate the cross-protective potential of our vaccine. Then, at week 10, the mice were sacrificed, and their spleens and lymph nodes were harvested to assess cellular responses. Flow cytometry was used to quantify the cellular response based on CD4+ and CD8+ expression, while the IgG response was measured using ELISA.
Results: After the IgG antibodies were measured, we observed a robust immune response in all groups, especially when compared to the control group (****p < 0.0001). These findings suggest that our vaccine not only elicited a humoral response against the CDCF62 strain but also against the other two strains. Analysis of the spleen CD8+ T cell responses revealed a significant increase in the percentage of CD8+ cells in groups C (*p < 0.05) and D (*p < 0.05) compared to the naïve group (A), while groups B (ns p >0.05) and E (ns p >0.05) did not show statistically significant differences from the naïve group. For the spleen CD4+ T cells, all vaccinated and challenged groups B(***p < 0.0002), C(****p < 0.0001), D(***p < 0.0002), E(*p < 0.05) exhibited a robust and significant increase in CD4+ cell percentages compared to the naïve group, with the highest responses observed in groups C and D. In the lymph nodes, CD8+ T cell responses were highest in group E (*p < 0.05), which was significantly elevated compared to the naïve group. CD4+ T cell responses in the lymph nodes were significantly higher in all vaccinated and challenged groups (B(*p < 0.05), C(**p < 0.0021), D (***p < 0.0002), E (****p < 0.0001)) compared to naïve group, with the strongest responses in groups D and E.
Conclusion: Overall, these results demonstrate that vaccination with the adjuvanted CDC F62 formulation could elicit a robust IgG response against all the strains of gonorrhea that were tested, induced strong CD4+ T cell responses in both spleen and lymph nodes, and could elicit cross-reactive cellular immunity against heterologous gonococcal strains, particularly when combined with adjuvant. CD8+ T cell responses were more modest but showed significant enhancement in select groups, supporting the potential of this approach for the development of a broad gonococcal vaccine.
References: 1. CDC Table 23. Gonorrhea — Reported Cases and Rates of Reported Cases by Age Group and Sex, United States Available online: https://www.cdc.gov/sti-statistics/data-vis/table-gc-agesex.html (accessed on 26 March 2025).
2. Greenberg, L.; Diena, B.B.; Ashton, F.A.; Wallace, R.; Kenny, C.P.; Znamirowski, R.; Ferrari, H.; Atkinson, J. Gonococcal Vaccine Studies in Inuvik. Can J Public Health 1974, 65, 29–33.
3. Gottlieb, S.L.; Deal, C.D.; Giersing, B.; Rees, H.; Bolan, G.; Johnston, C.; Timms, P.; Gray-Owen, S.D.; Jerse, A.E.; Cameron, C.E.; et al. The Global Roadmap for Advancing Development of Vaccines against Sexually Transmitted Infections: Update and next Steps. Vaccine 2016, 34, 2939–2947, doi:10.1016/j.vaccine.2016.03.111.
4. Bagwe, P.; Bajaj, L.; Gala, R.P.; D‘Souza, M.J.; Zughaier, S.M. Assessment of In Vitro Immunostimulatory Activity of an Adjuvanted Whole-Cell Inactivated Neisseria Gonorrhoeae Microparticle Vaccine Formulation. Vaccines 2022, 10, 983, doi:10.3390/vaccines10070983.
5. Jordan, P.W.; Snyder, L.A.; Saunders, N.J. Strain-Specific Differences in Neisseria Gonorrhoeae Associated with the Phase Variable Gene Repertoire. BMC Microbiol 2005, 5, 21, doi:10.1186/1471-2180-5-21.
6. Shafer, W.M.; Joiner, K.; Guymon, L.F.; Cohen, M.S.; Sparling, P.F. Serum Sensitivity of Neisseria Gonorrhoeae: The Role of Lipopolysaccharide. Journal of Infectious Diseases 1984, 149, 175–183, doi:10.1093/infdis/149.2.175.
7. Liu, Y.; Islam, E.A.; Jarvis, G.A.; Gray-Owen, S.D.; Russell, M.W. Neisseria Gonorrhoeae Selectively Suppresses the Development of Th1 and Th2 Cells, and Enhances Th17 Cell Responses, through TGF-β-Dependent Mechanisms. Mucosal Immunology 2012, 5, 320–331, doi:10.1038/mi.2012.12.
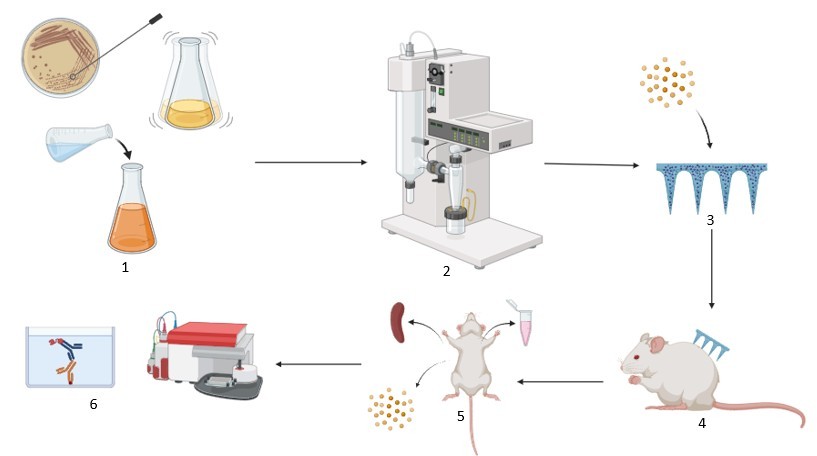 A graphical representation of the methodology of the study. 1. Growth and Inactivation of bacteria 2. Spray drying of inactivated bacteria into microparticles. 3. Loading microparticles into microneedle patches. 4. Administration of the vaccine to the mice. 5. Sacrifice and serum and organ collection from the mice. 5. ELISA analysis of serum samples and Flow cytometry analysis of organ samples.
A graphical representation of the methodology of the study. 1. Growth and Inactivation of bacteria 2. Spray drying of inactivated bacteria into microparticles. 3. Loading microparticles into microneedle patches. 4. Administration of the vaccine to the mice. 5. Sacrifice and serum and organ collection from the mice. 5. ELISA analysis of serum samples and Flow cytometry analysis of organ samples. .jpg) Total Serum IgG Levels
Total Serum IgG Levels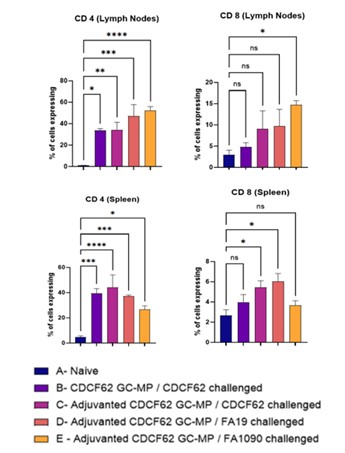 Percentage of cells in the spleen and lymph node populations expressing CD4 and CD8 markers.
Percentage of cells in the spleen and lymph node populations expressing CD4 and CD8 markers. 